All Photos(1)
About This Item
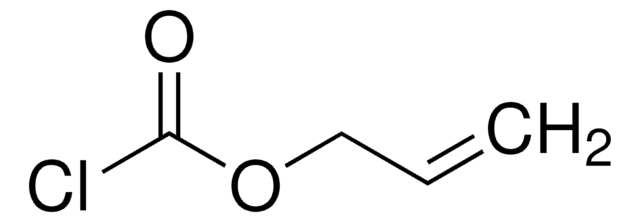
Linear Formula:
ClCH2COOC(CH3)3
CAS Number:
Molecular Weight:
150.60
Beilstein:
1753006
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.423 (lit.)
bp
48-49 °C/11 mmHg (lit.)
density
1.053 g/mL at 25 °C (lit.)
functional group
chloro
ester
SMILES string
CC(C)(C)OC(=O)CCl
InChI
1S/C6H11ClO2/c1-6(2,3)9-5(8)4-7/h4H2,1-3H3
InChI key
KUYMVWXKHQSIAS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
tert-Butyl chloroacetate was used in the synthesis of:
- imidazol-1-yl-acetic acid hydrochloride
- cis-disubstituted aziridine ester via aza-Darzens reaction
- 1,10-diaza-18-crown-6 based sensors bearing a coumarin fluorophore
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2
WGK
WGK 1
Flash Point(F)
114.8 °F
Flash Point(C)
46 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Substituent dependent fluorescence response of diazacrown-based PET sensors.
Nagy K, et al.
Tetrahedron, 64(27), 6191-6195 (2008)
Santosh Kumar Singh et al.
Beilstein journal of organic chemistry, 4, 42-42 (2008-12-24)
A convenient and practical synthesis of imidazol-1-yl-acetic acid hydrochloride was achieved via N-alkylation of imidazole using tert-butyl chloroacetate followed by a non-aqueous ester cleavage of the resulting imidazol-1-yl-acetic acid tert-butyl ester in the presence of titanium tetrachloride. The synthesized imidazol-1-yl-acetic
E Vedejs et al.
The Journal of organic chemistry, 65(18), 5498-5505 (2000-09-02)
Intermolecular alkylation of the aziridinyl oxazole 20 using PhSO(2)CH(2)CH(2)OTf is possible despite the presence of potentially nucleophilic aziridine nitrogen. The resulting oxazolium salt 22 reacts with BnNMe(3)(+)CN(-) to produce the azomethine ylide 24b via electrocyclic ring opening of an oxazoline
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service