All Photos(1)
About This Item
Empirical Formula (Hill Notation):
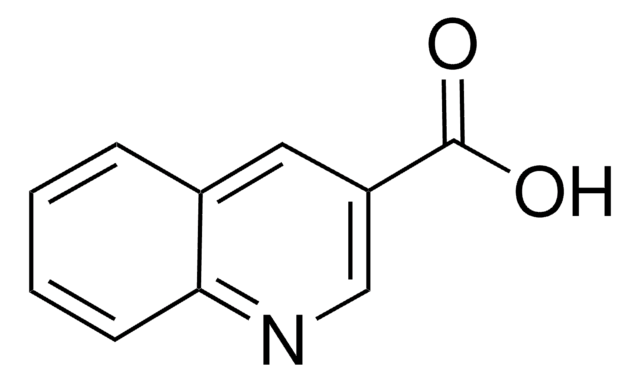
C10H7NO2
CAS Number:
Molecular Weight:
173.17
Beilstein:
5224
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
254-255 °C (lit.)
functional group
carboxylic acid
SMILES string
OC(=O)c1ccnc2ccccc12
InChI
1S/C10H7NO2/c12-10(13)8-5-6-11-9-4-2-1-3-7(8)9/h1-6H,(H,12,13)
InChI key
VQMSRUREDGBWKT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
4-Quinolinecarboxylic acid was used in the coupling reaction with diamine linker. A 4-quinolinecarboxylic acid analogue, brequinar sodium was used to inhibit dihydroorotate dehydrogenase and the de novo biosynthesis of pyrimidine.
Biochem/physiol Actions
4-Quinolinecarboxylic acid showed anti-tumor activity against L1210 leukemia and B16 melanoma.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
He Huang et al.
The Journal of organic chemistry, 74(15), 5476-5480 (2009-07-04)
We developed a simple and convenient copper-catalyzed method for the synthesis of quinoline-2-carboxylate derivatives through sequential intermolecular addition of alkynes onto imines and subsequent intramolecular ring closure by arylation. The efficiency of this system allowed the reactions to be carried
Solid-phase synthesis of substituted quinoline and isoquinoline derivatives using heterocyclic N-oxide chemistry.
Tetrahedron Letters, 39(27), 4749-4752 (1998)
D L Dexter et al.
Cancer research, 45(11 Pt 1), 5563-5568 (1985-11-01)
A novel, substituted 4-quinolinecarboxylic acid (NSC 339768) demonstrated antitumor activity against L1210 leukemia and B16 melanoma in the National Cancer Institute's Developmental Therapeutics Program. An extensive analogue synthesis program was initiated; over 200 derivatives were synthesized and tested for anticancer
Afshin Zarghi et al.
Bioorganic & medicinal chemistry, 17(14), 5312-5317 (2009-06-30)
A group of 4-carboxyl quinoline derivatives possessing a methylsulfonyl COX-2 pharmacophore at the para position of the C-2 phenyl ring were designed and synthesized as selective COX-2 inhibitors. In vitro COX-1/COX-2 structure-activity relationships were determined by varying the substituents on
The cardiovascular and respiratory effects of 3-hydroxy-2-phenyl cinchoninic acid.
H A WALKER et al.
The Journal of pharmacology and experimental therapeutics, 102(2), 71-78 (1951-06-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service