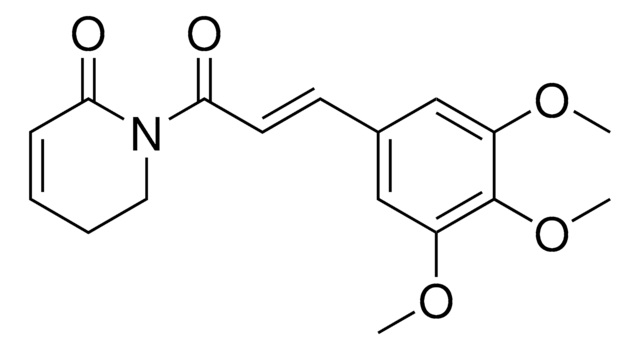
SMB01055
Colladin
≥90% (LC/MS-ELSD)
Synonym(s):
Coladin
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C26H32O5
CAS Number:
Molecular Weight:
424.53
UNSPSC Code:
12352205
NACRES:
NA.25
Recommended Products
biological source
plant
Assay
≥90% (LC/MS-ELSD)
form
solid
mol wt
424.53
solubility
water: slightly soluble
application(s)
metabolomics
vitamins, nutraceuticals, and natural products
storage temp.
−20°C
General description
Colladin, a sesquiterpenoid coumarin, is a natural product commonly available from Ferula Sp. (F. arrigonii, F. vesceritensis, F. campestris and F. sinaica) plants. Existing research suggests that this plant-derived metabolite exerts various biological activities, including anticancer, neuroprotective, and antiglaucoma properties.
Application
It is a natural product derived from plant source that finds application in compound screening libraries, metabolomics, phytochemical, and pharmaceutical research.
Biochem/physiol Actions
According to the existing research, Coladin exhibited notable anti-cancer properties by substantially reducing cell proliferation and mitochondrial dehydrogenase activity in mouse B16F1 melanoma cells. These effects induced apoptosis through mechanisms involving decreased mitochondrial membrane potential and mitochondrial respiratory rate, underscoring its potential as a natural anti-cancer agent. Isolated from the roots of Ferulago campestris, coladin, along with other compounds such as umbelliprenin and epielmanticine, displayed inhibitory activity against Acetylcholinesterase (AChE) with an IC50 of 0.1 mM, suggesting its potential application in the treatment of neurological disorders like Alzheimer′s disease. Coladin, identified as one of the constituents in Heptaptera triquetra fruit, exhibited remarkable inhibitory activity against acetylcholinesterase (AChE), human carbonic anhydrase isoenzyme (hCA) I, and hCA II, with IC50 values of 8.25 nM, 28.90 nM, and 43.31 nM, respectively. This highlights its potential in the development of phytotherapeutics for conditions like Alzheimer′s disease (AD) and glaucoma.
Features and Benefits
- High quality compound suitable for multiple research applications
- Compatible with HPLC and mass spectrometry techniques
Other Notes
For additional information on our range of Biochemicals, please complete this form.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Identification of non-alkaloid acetylcholinesterase inhibitors from Ferulago campestris (Besser) Grecescu (Apiaceae)
Dall'Acqua S, et al.
Fitoterapia, 81, 1208-1212 (2010)
Phytochemical content and enzyme inhibitory effect of Heptaptera triquetra (Vent.) Tutin fruit against acetylcholinesterase and carbonic anhydrase I and II isoenzymes
Kaya AC, et al.
Chemical Papers, 77, 5829?5837-5829?5837 (2023)
Sesquiterpene esters and sesquiterpene coumarin ethers from Ferula linkii-TF
Gonzalez A G, et al.
Phytochemistry, 33(4), 863-866 (1993)
Cytotoxicity of sesquiterpenes ferulenol and coladin on liver FAO and B16F1 melanoma cells
Boulmeltout M, et al.
Pharmacognosy magazine, 14, 333-337 (2018)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service