17790
β-Eudesmol
≥90% (GC)
Synonym(s):
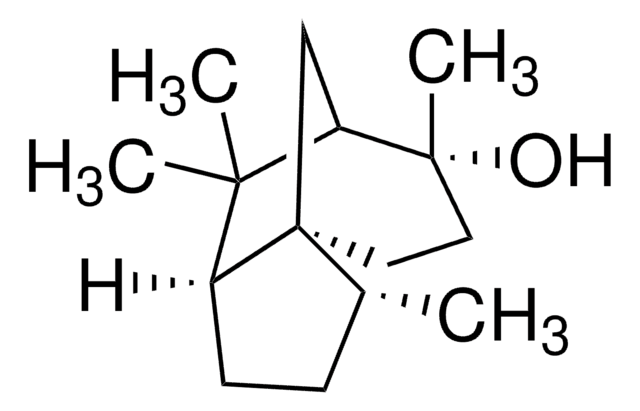
(2R,4aR,8aS)-Decahydro-8-methylene-α,α,4a-trimethyl-2-naphthylmethanol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C15H26O
CAS Number:
Molecular Weight:
222.37
Beilstein:
5735560
MDL number:
UNSPSC Code:
12352212
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Assay
≥90% (GC)
form
powder
mp
72-74 °C (lit.)
application(s)
metabolomics
vitamins, nutraceuticals, and natural products
storage temp.
2-8°C
SMILES string
[H][C@@]12C[C@@H](CC[C@@]1(C)CCCC2=C)C(C)(C)O
InChI
1S/C15H26O/c1-11-6-5-8-15(4)9-7-12(10-13(11)15)14(2,3)16/h12-13,16H,1,5-10H2,2-4H3/t12-,13+,15-/m1/s1
InChI key
BOPIMTNSYWYZOC-VNHYZAJKSA-N
General description
β-Eudesmol is an aromatic, oxygenated sesquiterpene compound. It is primarily observed in the bark of mangolia trees and can also be extracted from other medical herbs such as Cryptomeria japonica, Atractylodes lancea, Pterocarpus santalinus, Ginkgo biloba, and Nardostachys jatamansi.
Application
β-Eudesmol has been used:
- as a treatment to test its antioxidant, anti-inflammatory, cell preservation effects on human dermal fibroblasts (HDFs)
- as a reference standard to investigate if metabolically engineered E. coli extracted terpene synthase (Tps) genes encode for β-eudesmol synthase by using gas chromatography-mass spectrometry (GC/MS) analysis
- to test its antiviral effects against herpes simplex virus type 1 (HSV-1)
- to test its reversal effects of cocaine-induced planarian behavior
Biochem/physiol Actions
β-Eudesmol has many pharmacological benefits. It is an active ingredient present in many essential oils, showing antioxidant and antimicrobial activities. β-Eudesmol elicits its antifungal and anti-wood-decay fungal activities in leaf essential oil of Litsea coreana tree, and the twigs of Taiwania cryptomerioides trees respectively. It suppresses tumor cell proliferation, growth, and migration of human tumor cells. β-Eudesmol is known to have various protective effects on the nervous system.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chen-Lung Ho et al.
Natural product communications, 5(7), 1143-1146 (2010-08-26)
The hydrodistillated leaf essential oil of Machilus pseudolongifolia was analyzed to determine its composition and yield. Seventy compounds were identified, the main components being beta-eudesmol (26.8%), alpha-cadinol (20.8%), viridiflorene (8.9%), alpha-caryophyllene (5.3%), globulol (4.6%) and beta-caryophyllene (4.2%). Oxygenated sesquiterpenes (60.1%)
Chen-Lung Ho et al.
Natural product communications, 5(10), 1677-1680 (2010-12-03)
The hydrodistilled leaf essential oil of Litsea coreana was analyzed by GC/FID and GC/MS. Fifty-two compounds were identified, the main components being n-decanal (27.5%), 2E,6E-farnesol (25.8%), beta-eudesmol (10.3%), ethyl n-dodecanoate (8.0%) and tau-cadinol (6.6%). Oxygenated sesquiterpenes (56.8%) and non-terpenoids (37.0%)
Antimicrobial and antioxidant activities and gas chromatography mass spectrometry (GC/MS) analysis of the essential oils of Ajuga bracteosa Wall. ex Benth. and Lavandula dentata L. growing wild in Yemen
Mothana RA, et al.,
Journal of Medicinal Plants Research, 6(15), 3066-3071 (2012)
Jean Waikedre et al.
Chemistry & biodiversity, 9(3), 644-653 (2012-03-17)
Mortality due to fungal infections has increased substantially, becoming a worldwide problem in public health. As a contribution to the discovery of new antifungal agents, the properties of the heartwood essential oils of two trees growing in New Caledonia, Callitris
Yanchun Li et al.
Phytotherapy research : PTR, 27(3), 338-343 (2012-05-16)
β-eudesmol, a natural sesquiterpenol present in a variety of Chinese herbs, is known to inhibit the proliferation of human tumor cells. However, the molecular mechanisms of the effect of β-eudesmol on human tumor cells are unknown. In the present study
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service