All Photos(1)
About This Item
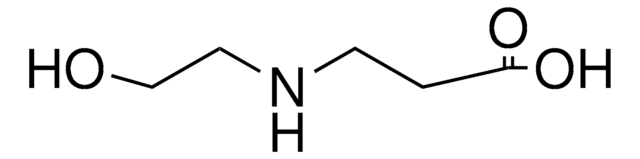
Empirical Formula (Hill Notation):
C4H9NO2
CAS Number:
Molecular Weight:
103.12
Beilstein:
1720922
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0% (TLC)
form
powder
reaction suitability
reaction type: solution phase peptide synthesis
color
colorless to white
application(s)
peptide synthesis
SMILES string
CN[C@@H](C)C(O)=O
InChI
1S/C4H9NO2/c1-3(5-2)4(6)7/h3,5H,1-2H3,(H,6,7)/t3-/m0/s1
InChI key
GDFAOVXKHJXLEI-VKHMYHEASA-N
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Z Grzonka et al.
International journal of peptide and protein research, 25(4), 375-381 (1985-04-01)
The 600 MHz proton n.m.r. spectra of (sarcosyl7)-oxytocin and (N-methylalanyl7) oxytocin in 2H2O solution have been recorded and completely assigned. In each case the spectrum indicates the presence of two slowly interconverting conformers, which are the cis-trans isomers about the
D Gazis et al.
International journal of peptide and protein research, 23(1), 78-83 (1984-01-01)
Substituting sarcosine or N-methylalanine for proline in the inhibitory vasopressin analogs dPAVP and d(CH2)5AVP had the following effects: 1) milk ejection and antidiuretic activities were severely depressed, 2) pressor antagonism was maintained but weakened somewhat, and 3) antagonism in the
C H Tan et al.
Biochemical pharmacology, 39(5), 955-958 (1990-03-01)
Synaptosomes isolated from adult rat cerebral cortices were used for studying the uptake of L-leucine by the Na(+)-dependent route. Three non-metabolizable amino acid analogues, which had been used previously to discriminate the Na(+)-dependent A-type uptake system of animal cells, were
Z Grzonka et al.
Journal of medicinal chemistry, 26(4), 555-559 (1983-04-01)
Eight analogues of oxytocin and arginine-vasopressin were synthesized, in which the proline residue in position 7 was replaced by either sarcosine or N-methylalanine; some of the pharmacological properties of these analogues were evaluated. In peptides containing a beta-mercaptopropionic acid residue
Zarixia Zavala-Ruiz et al.
The Journal of biological chemistry, 278(45), 44904-44912 (2003-09-04)
Crystal structures of the class II major histocompatibilty complex (MHC) protein, HLA-DR1, generally show a tight fit between MHC and bound peptide except in the P6/P7 region of the peptide-binding site. In this region, there is a shallow water-filled pocket
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service