N9608
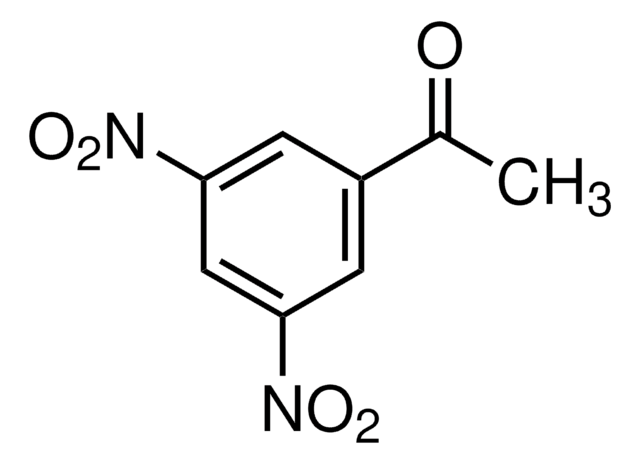
4′-Nitroacetophenone
98%
Synonym(s):
1-(4-Nitrophenyl)ethan-1-one, 1-(4-Nitrophenyl)ethanone, 1-Acetyl-4-nitrobenzene, 4-Nitrophenyl methyl ketone, 4′-Nitroacetophenone, p-Acetylnitrobenzene
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
O2NC6H4COCH3
CAS Number:
Molecular Weight:
165.15
Beilstein:
607777
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
crystals
bp
202 °C (lit.)
mp
75-78 °C (lit.)
SMILES string
CC(=O)c1ccc(cc1)[N+]([O-])=O
InChI
1S/C8H7NO3/c1-6(10)7-2-4-8(5-3-7)9(11)12/h2-5H,1H3
InChI key
YQYGPGKTNQNXMH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chemical radiosensitization of anoxic mammalian cells: effect of cell concentration.
D A Agnew et al.
Radiation research, 57(2), 246-259 (1974-02-01)
The effect of combinations of nitroaromatic and nitroxyl radiosensitizers on the radiation survival response of Chinese hamster cells, V.79-753B, in vitro.
B C Millar et al.
Radiation research, 88(2), 369-376 (1981-11-01)
Modification of radiation sensitivity of Bacillus megaterium spores by N2O and p-nitroacetophenone.
D Ewing et al.
Radiation research, 58(3), 481-488 (1974-06-01)
Katryn L Williams et al.
Journal of agricultural and food chemistry, 66(22), 5462-5472 (2018-05-15)
Benzobicyclon [3-(2-chloro-4-(methylsulfonyl)benzoyl)-2-phenylthiobicyclo[3.2.1]oct-2-en-4-one] is a pro-herbicide used against resistant weeds in California rice fields. Persistence of its active product, benzobicyclon hydrolysate, is of concern. As an acidic herbicide, the neutral species photolyzed faster than the more predominant anionic species ( t1/2
K Tatsumi et al.
Journal of pharmacobio-dynamics, 4(2), 101-108 (1981-02-01)
The electron transfer mechanism in the reduction of aromatic nitro compounds by xanthine oxidase was investigated using methyl p-nitrobenzoate and p-nitroacetophenone as substrates. Methyl p-nitrobenzene was reduced by both one-electron and more than two-electron transfer mechanisms in the enzyme-electron donor
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service