All Photos(1)
About This Item
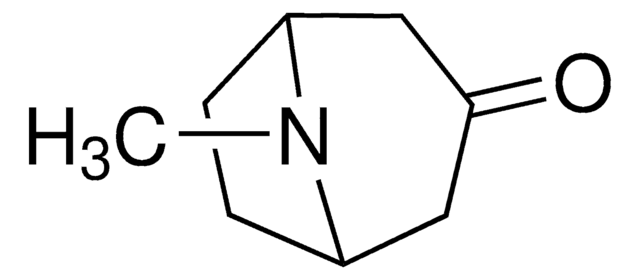
Empirical Formula (Hill Notation):
C8H15NO
CAS Number:
Molecular Weight:
141.21
Beilstein:
80188
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97.0% (NT)
form
powder
impurities
0-3% water
solubility
H2O: 0.1 g/mL, clear
storage temp.
2-8°C
SMILES string
CN1[C@H]2CC[C@@H]1C[C@H](O)C2
InChI
1S/C8H15NO/c1-9-6-2-3-7(9)5-8(10)4-6/h6-8,10H,2-5H2,1H3/t6-,7+,8+
InChI key
CYHOMWAPJJPNMW-JIGDXULJSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Interference of mandelic acid with the determination of homatropine hydrobromide by second-order derivative spectroscopy.
J S Millership
Journal of pharmaceutical and biomedical analysis, 12(9), 1199-1203 (1994-09-01)
Gábor Maksay et al.
Bioorganic & medicinal chemistry, 16(4), 2086-2092 (2007-12-07)
(Hetero)aromatic mono- and diesters of tropine and nortropine were prepared. Modulation of [3H]strychnine binding to glycine receptors of rat spinal cord was examined with a ternary allosteric model. The esters displaced [3H]strychnine binding with nano- or micromolar potencies and strong
B A Bartholomew et al.
The Biochemical journal, 307 ( Pt 2), 603-608 (1995-04-15)
Tropine dehydrogenase was induced by growth of Pseudomonas AT3 on atropine, tropine or tropinone. It was NADP(+)-dependent and gave no activity with NAD+. The enzyme was very unstable but a rapid purification procedure using affinity chromatography that gave highly purified
Roland Molinié et al.
Rapid communications in mass spectrometry : RCM, 23(24), 4031-4037 (2009-11-20)
N-Demethylation of tropine is an important step in the degradation of this compound and related metabolites. With the purpose of understanding the reaction mechanism(s) involved, it is desirable to measure the 15N kinetic isotope effects (KIEs), which can be accessed
Grit Rothe et al.
Journal of experimental botany, 54(390), 2065-2070 (2003-07-30)
Putrescine N-methyltransferase (PMT) is the first alkaloid-specific enzyme for nicotine and tropane alkaloid formation. The pmt gene from Nicotiana tabacum was fused to the CaMV 35S promoter and integrated into the Atropa belladonna genome. Transgenic plants and derived root cultures
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service