917230
NanoFabTx™ device accessory
interface H
Synonym(s):
Microfluidic kit, NanoFabTx™, Nanoformulation
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
UNSPSC Code:
41121800
NACRES:
NA.23
Recommended Products
description
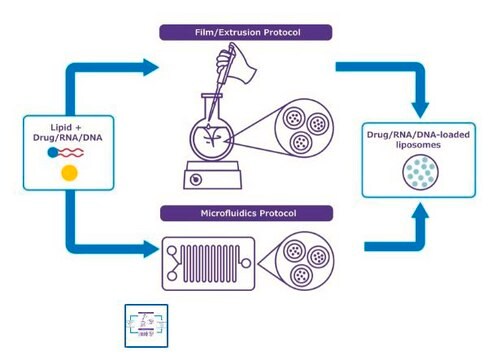
Microfludic hardware kit component
Kit components : Inerface H x 1
Quality Level
application(s)
advanced drug delivery
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
Legal Information
NANOFABTX is a trademark of Sigma-Aldrich Co. LLC
related product
Product No.
Description
Pricing
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Samar Damiati et al.
Genes, 9(2) (2018-02-22)
Microfluidic devices present unique advantages for the development of efficient drug carrier particles, cell-free protein synthesis systems, and rapid techniques for direct drug screening. Compared to bulk methods, by efficiently controlling the geometries of the fabricated chip and the flow
Xuanyu Li et al.
Advanced drug delivery reviews, 128, 101-114 (2017-12-27)
Microfluidic chips allow the rapid production of a library of nanoparticles (NPs) with distinct properties by changing the precursors and the flow rates, significantly decreasing the time for screening optimal formulation as carriers for drug delivery compared to conventional methods.
Andrew Gdowski et al.
Journal of nanobiotechnology, 16(1), 12-12 (2018-02-13)
The process of optimization and fabrication of nanoparticle synthesis for preclinical studies can be challenging and time consuming. Traditional small scale laboratory synthesis techniques suffer from batch to batch variability. Additionally, the parameters used in the original formulation must be
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service