All Photos(2)
About This Item
Empirical Formula (Hill Notation):
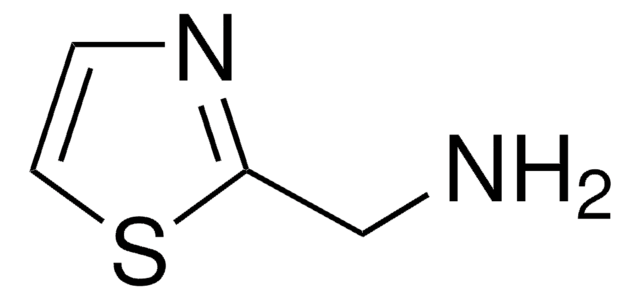
C4H6N2S
CAS Number:
Molecular Weight:
114.17
Beilstein:
109603
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
mp
93-98 °C (lit.)
SMILES string
Cc1cnc(N)s1
InChI
1S/C4H6N2S/c1-3-2-6-4(5)7-3/h2H,1H3,(H2,5,6)
InChI key
GUABFMPMKJGSBQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Amino-5-methylthiazole is a heterocyclic building block. It is one of the major alkaline metabolite of tenoxicam (TX) and meloxicam (MX).
Application
2-Amino-5-methylthiazole may be used in the preparation of acrylamide monomer, 5-methyl-2-thiozyl methacrylamide (MTMAAm).
2-Amino-5-methylthiazole may be used in the preparation of mixed-ligand dien-Cu(II) complexes (dien=diethylenetriamine). It may be used in the preparation of a new series of Schiff mono/dibase coordination compounds, having potential anticancer and antibacterial activities.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - STOT RE 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Riccardo Baron et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 9(7), 983-988 (2008-04-18)
The binding of 2-amino-5-methylthiazole to the W191G cavity mutant of cytochrome c peroxidase is an ideal test case to investigate the entropic contribution to the binding free energy due to changes in receptor flexibility. The dynamic and thermodynamic role of
A Th Chaviara et al.
Journal of inorganic biochemistry, 99(2), 467-476 (2004-12-29)
Two novel mononuclear Cu(II) coordination compounds of the type [Cu(dptaS)Cl(2)] and [Cu(dptaS)Br(2)] (dptaS=1,3-propanediamine, N(1)-[3-aminopropyl]-N(3)-[2-thienylmethylidene] or Schiff mono-base of dipropylenetriamine with 2-thiophene-carboxaldehyde) were prepared by the hydrolysis of the di-bases, [Cu(dptaSS)Cl(2)] and [Cu(dptaSS)Br(2)] (dptaSS=1,3-propanediamine, N(1)-[2-thienylmethylidene]-N(3)-[[2-thienylmethylidene]aminopropyl] or Schiff di-base of dipropylenetriamine with
A Th Chaviara et al.
Journal of inorganic biochemistry, 98(8), 1271-1283 (2004-07-24)
A new series of coordination compounds of the starting materials [Cu(dienX(2)Y(2))] and their adducts [Cu(dienXXY(2))(2a-5mt)] (where dien=diethylenetriamine, dienXX=Schiff bases of diethylenetriamine with 2-furaldehyde or 2-thiophene-carboxaldehyde, X=O, S, Y=Cl, Br, NO(3) and 2a-5mt=2-amino-5-methylthiazole) were synthesized by stepwise reactions and their structures
E Pontiki et al.
Journal of enzyme inhibition and medicinal chemistry, 23(6), 1011-1017 (2008-11-14)
Highly reactive radicals are implicated in many pathological conditions. The quest for radical scavengers or antioxidants, spans the previous decades. A new series of complexes of the type [Cu (dien) (2a-2tzn) Y(2)] and [Cu (dienXXY(2)) (2a-5mt)] and of the type
Ratio derivative spectrophotometric method for the determination of some oxicams in presence of their alkaline degradation products.
Taha EA, et al.
Scientia Pharmaceutica, 71(4), 303-320 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service