T82805
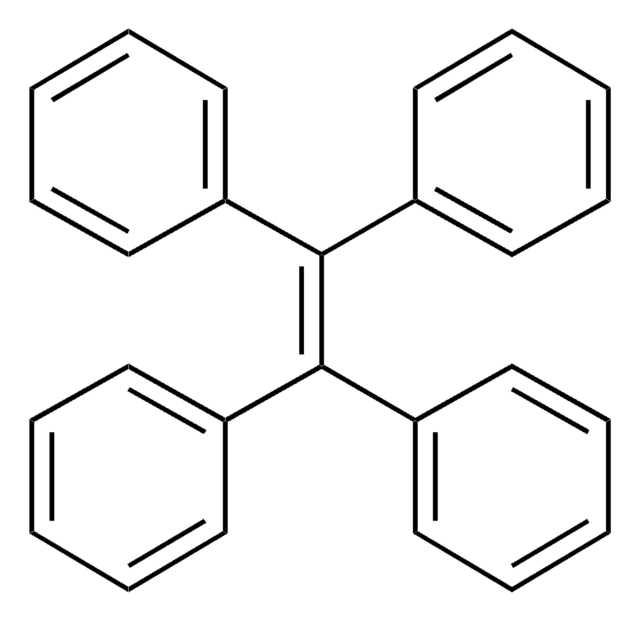
Triphenylethylene
99%
Synonym(s):
1,1,2-Triphenylethene, 1,1,2-Triphenylethylene, 1,1′,1′′-(1-Ethenyl-2-ylidene)tris[benzene], 1,2-Diphenylethenylbenzene, Benzilidenediphenylmethane, Ethene-1,1,2-triyltribenzene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5CH=C(C6H5)2
CAS Number:
Molecular Weight:
256.34
Beilstein:
1867462
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
69-71 °C (lit.)
SMILES string
c1ccc(cc1)\C=C(/c2ccccc2)c3ccccc3
InChI
1S/C20H16/c1-4-10-17(11-5-1)16-20(18-12-6-2-7-13-18)19-14-8-3-9-15-19/h1-16H
InChI key
MKYQPGPNVYRMHI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
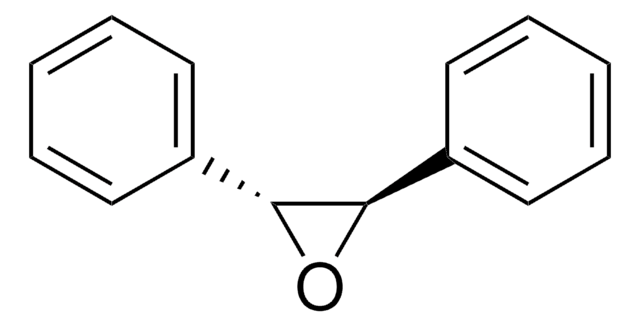
Triphenylethylene is an aromatic hydrocarbon, which can be used as a starting material to prepare 2,2,3-triphenyloxirane by asymmetric epoxidation reaction using fluorous chiral manganese complex as a catalyst. It is also used to prepare dihydro-4,5,5-triphenyl-2(3H)-furanone by reacting with acetic anhydride in the presence of MnO2 and NaOAc.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Xiao-Fang Li et al.
Journal of fluorescence, 21(5), 1969-1977 (2011-05-24)
New aggregation-induced emission (AIE) compounds derived from triphenylethylene were synthesized. The thermal, photophysical, electrochemical and aggregation-induced emissive properties were investigated. All the compounds had strong blue light emission capability and good thermal stability. Their maximum fluorescence emission wavelengths were between
Effect of estrogenic and antiestrogenic triphenylethylene derivatives on progesterone and estrogen receptors levels of MCF-7 cells.
N Legros et al.
Biochemical pharmacology, 42(9), 1837-1841 (1991-10-09)
M Pons et al.
Journal of steroid biochemistry, 36(5), 391-397 (1990-08-14)
The relative binding affinities of a series of twelve para-hydroxylated triphenylethylenes (TPEs) for the estradiol receptor (ER) of calf uterus cytosol were measured by a competition method. The results obtained under equilibrium conditions support the hypothesis of the additivity of
[The search for active antitumor agents among triphenylethylene derivatives].
P V Sergeev et al.
Voprosy onkologii, 41(2), 49-49 (1995-01-01)
MnO 2-promoted carboesterification of alkenes with anhydrides: a facile approach to ?-lactones
Wu Lihuan, et al.
Chemical Communications (Cambridge, England), 52(12), 2628-2631 (2016)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service