All Photos(1)
About This Item
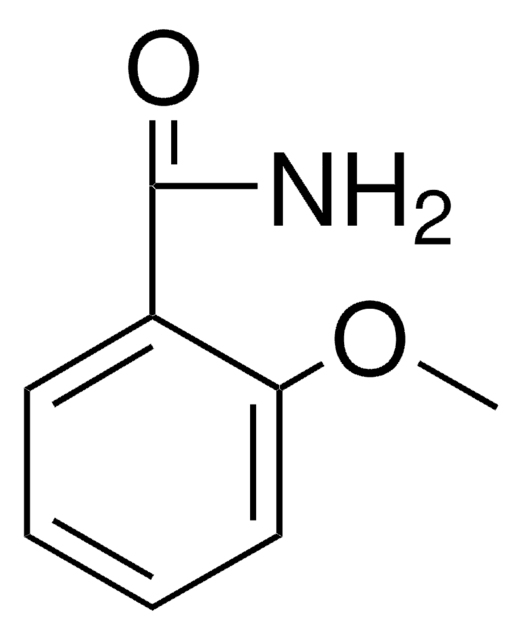
Linear Formula:
CH3OC6H4CONH2
CAS Number:
Molecular Weight:
151.16
Beilstein:
2206857
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
132.5-135.5 °C (lit.)
SMILES string
COc1cccc(c1)C(N)=O
InChI
1S/C8H9NO2/c1-11-7-4-2-3-6(5-7)8(9)10/h2-5H,1H3,(H2,9,10)
InChI key
VKPLPDIMEREJJF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
B C Waldman et al.
Nucleic acids research, 18(20), 5981-5988 (1990-10-25)
We determined the effect of 3-methoxybenzamide (3-MB), a competitive inhibitor of poly(ADP-ribose)polymerase (E.C. 2.4.2.30), on illegitimate and extrachromosomal homologous recombination in mouse Ltk- cells. Cells were transfected with a wild type Herpes thymidine kinase (tk) gene or with two defective
Neil R Stokes et al.
Antimicrobial agents and chemotherapy, 57(1), 317-325 (2012-11-02)
The bacterial cell division protein FtsZ is an attractive target for small-molecule antibacterial drug discovery. Derivatives of 3-methoxybenzamide, including compound PC190723, have been reported to be potent and selective antistaphylococcal agents which exert their effects through the disruption of intracellular
H Y Dong et al.
Mutation research, 331(2), 197-204 (1995-10-01)
To investigate the origin of DNA repair in rat pleural mesothelial cells (RPMC) exposed to asbestos fibers, poly(ADP-ribose) polymerase (PARP) activity was measured in the asbestos-treated cells. As bleomycin has been shown to activate poly(ADP-ribose) synthesis in several cell systems
S Hauschildt et al.
Advances in experimental medicine and biology, 419, 249-252 (1997-01-01)
Stimulating monocytes/macrophages with bacterial lipopolysaccharide (LPS) results in TNF-alpha, IL-1, IL-6 and nitrite (NO2-) formation. Inhibitors of poly(ADP-ribose)polymerase inhibit release of these mediators by preventing mRNA expression indicating that ADP-ribosylation plays a crucial role in the synthesis of these mediators.
N Nemoto et al.
Carcinogenesis, 12(4), 623-629 (1991-04-01)
Regulation of P(1)450 gene expression in mouse hepatocytes from responsive (C57BL/6) and non-responsive (DBA/2) strains in primary culture was investigated with respect to aryl hydrocarbon hydroxylase (AHH) activity and P450 transcript levels. Although significant induction of AHH activity in C57BL/6
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service