909459
Fluorinated VHL Spy Molecule 4
≥98%
Synonym(s):
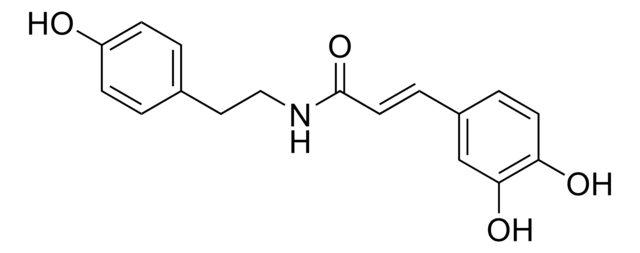
(2S,4R)-N-(4-bromobenzyl)-4-hydroxy-1-(3,3,3-trifluoropropanoyl)pyrrolidine-2-carboxamide
About This Item
Recommended Products
ligand
VH032 derivative
Assay
≥98%
form
powder
SMILES string
O=C([C@@H]1C[C@@H](O)CN1C(CC(F)(F)F)=O)NCC2=CC=C(Br)C=C2
Application
The E3 ligase VHL is of growing interest for targeted protein degradation, and these spy molecules will facilitate the identification of novel VHL ligands and VHL-based degraders.
Other Notes
Structure-guided design and optimization of small molecules targeting the protein-protein interaction between the von Hippel-Lindau (VHL) E3 ubiquitin ligase and the hypoxia inducible factor (HIF) alpha subunit with in vitro nanomolar affinities
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Related Content
The Ciulli group are broadly interested with understanding and exploiting the ligandability of protein-protein interactions (PPIs) and protein surfaces within complex biological systems using chemical and structural cell biology approaches. Current research efforts are directed towards targeting PPIs molecular recognition within protein families of biological and medical relevance within the Ubiquitin and Chromatin systems by developing small molecules that disrupt PPIs and that induce targeted protein degradation (PROTAC®) - as tools to probe biology and leads for drug discovery.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service