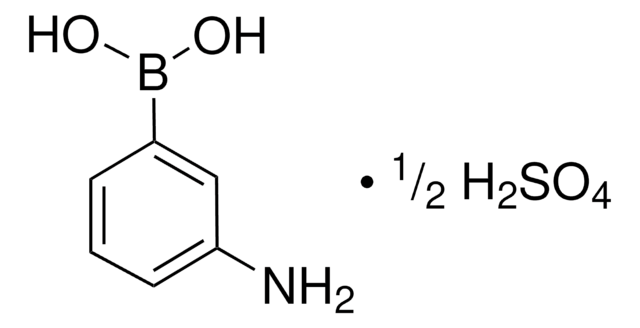
566012
3-Acetamidophenylboronic acid
≥95%
Synonym(s):
3-Acetamidobenzeneboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3CONHC6H4B(OH)2
CAS Number:
Molecular Weight:
178.98
Beilstein:
3278316
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95%
form
powder
mp
135 °C (lit.)
functional group
amide
SMILES string
CC(=O)Nc1cccc(c1)B(O)O
InChI
1S/C8H10BNO3/c1-6(11)10-8-4-2-3-7(5-8)9(12)13/h2-5,12-13H,1H3,(H,10,11)
InChI key
IBTSWKLSEOGJGJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Reactant involved in:
Reactant involved in the synthesis of a variety of inhibitors including:
- Suzuki-Miyaura coupling reactions
- Trifluoromethylation
Reactant involved in the synthesis of a variety of inhibitors including:
- NR2B subtype of NMDA receptor antagonists for antidepressant activity
- Biphenylylmethylimidazole derivatives for use as 17,20-lyase inhibitors
- (Indolyl)-3,5-substituted benzene analogs with antimitotic and antitumor activity
- Substituted pyrrolidines and tetrahydrofurans as AMPA receptor positive modulators
Other Notes
Contains varying amounts of anhydride.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
André J van der Vlies et al.
Bioconjugate chemistry, 30(3), 861-870 (2019-01-25)
Curcumin (Cur) has a wide range of bioactivities that show potential for the treatment of cancer as well as chronic diseases associated with inflammation and aging. However, the therapeutic efficacy of Cur has been hampered by its rapid degradation under
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service