All Photos(2)
About This Item
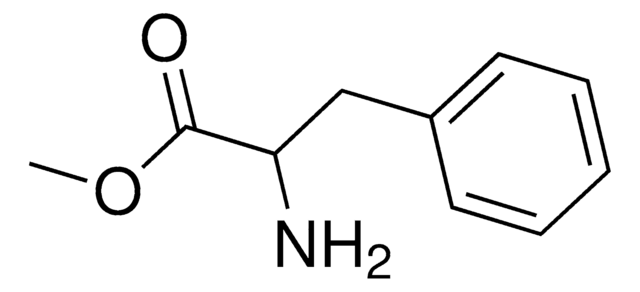
Linear Formula:
C6H5CH2CH(NH2)CO2CH3 · HCL
CAS Number:
Molecular Weight:
215.68
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
reaction suitability
reaction type: solution phase peptide synthesis
mp
159-163 °C (lit.)
application(s)
peptide synthesis
storage temp.
2-8°C
SMILES string
Cl[H].COC(=O)[C@H](N)Cc1ccccc1
InChI
1S/C10H13NO2.ClH/c1-13-10(12)9(11)7-8-5-3-2-4-6-8;/h2-6,9H,7,11H2,1H3;1H/t9-;/m1./s1
InChI key
SWVMLNPDTIFDDY-SBSPUUFOSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Shotaro Tsuchiyama et al.
Biotechnology progress, 23(4), 820-823 (2007-05-08)
The PST-01 protease is a metalloprotease that has zinc ion at the active center and is very stable in the presence of water-soluble organic solvents. The reaction rates and the equilibrium yields of the aspartame precursor N-carbobenzoxy-L-aspartyl-L-phenylalanine methyl ester (Cbz-Asp-Phe-OMe)
J S Shin et al.
Biotechnology and bioengineering, 69(5), 577-583 (2000-07-18)
We recently demonstrated (J Am Chem Soc 121:3334-3340, 1999) that enzymatic enantioselectivity in organic solvents can be markedly enhanced by temporarily enlarging the substrate via salt formation. In the present study, this approach was expanded by finding that, in addition
G D Castro et al.
Research communications in molecular pathology and pharmacology, 98(1), 85-90 (1998-01-22)
Reaction mixtures containing phenylalanine methyl ester and thymine in pure carbon tetrachloride in the presence of benzoyl peroxide produced trichloromethyl and trichloromethylperoxyl free radicals which via hydrogen abstraction reactions sparked the formation of phenylalanine-thymine adducts, whose structures were elucidated by
Robert Rennert et al.
Biochimica et biophysica acta, 1758(3), 347-354 (2005-12-29)
Many promising therapeutics are currently awaiting their clinical application. Due to their low capability of cell membrane crossing, these compounds do not reach their site of action. One way to overcome this problem might be the fusion of these agents
L Ye et al.
Journal of molecular recognition : JMR, 11(1-6), 75-78 (1999-03-17)
We have studied the possibility of shifting a thermodynamically unfavourable enzymatic equilibrium towards product formation via the addition of a highly specific adsorbent. The commercially interesting enzymatic condensation of Z-L-aspartic acid with L-phenylalanine methyl ester to the sweetener aspartame was
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service