All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H8O3
CAS Number:
Molecular Weight:
116.12
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
refractive index
n20/D 1.418 (lit.)
bp
62-65 °C/32 mmHg (lit.)
density
1.097 g/mL at 25 °C (lit.)
functional group
ester
ether
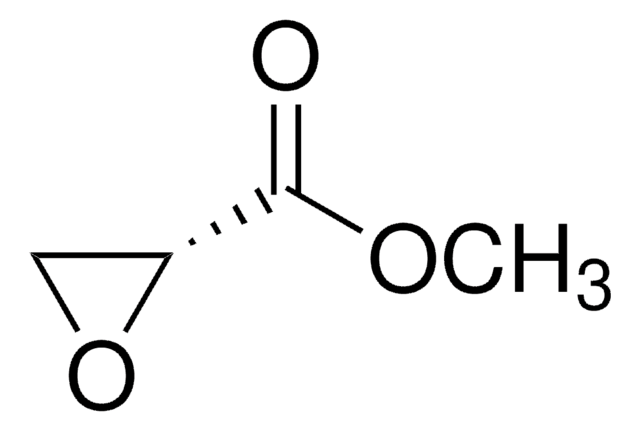
SMILES string
COC(=O)C1(C)CO1
InChI
1S/C5H8O3/c1-5(3-8-5)4(6)7-2/h3H2,1-2H3
InChI key
OSYXXQUUGMLSGE-UHFFFAOYSA-N
Related Categories
General description
Methyl 2-methylglycidate (methyl 2-methyloxirane-2-carboxylate) is an ester derived epoxide. It is reported to be formed by the reaction of methyl pyruvate with diazomethane. It is formed as an intermediate in the synthesis of 1,2,4-oxadiazole derivatives.
Application
Methyl 2-methylglycidate may be used in the synthesis of:
- 2-methyl-2-hydroxy-1-propanol
- 2,3-dihydroxy-2-methyl propionic acid
- methyl 2-hydroxy-3-((4-methoxyphenyl)sulfonyl)-2-methylpropanoate
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
129.2 °F - closed cup
Flash Point(C)
54 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
DABSO-Based, Three-Component, One-Pot Sulfone Synthesis.
Deeming AS, et al.
Organic Letters, 16(1), 150-153 (2013)
β-Keto phosphonic esters.
Arbuzov BA, et al.
Russian Chemical Bulletin, 12(4), 604-610 (1963)
Gopal L Khatik et al.
Bioorganic & medicinal chemistry letters, 22(5), 1912-1916 (2012-02-14)
Sulfide and sulfonyl derivatives of 1,2,4-oxadiazoles were synthesized and screened by MTT assay on the prostate cancer cells, DU-145. Six compounds were identified as potential anti-prostate cancer agents with IC(50) values ranging from 0.5 to 5.1μM. These compounds exhibited good
R J Steffan et al.
Applied and environmental microbiology, 63(11), 4216-4222 (1997-11-15)
Several propane-oxidizing bacteria were tested for their ability to degrade gasoline oxygenates, including methyl tert-butyl ether (MTBE), ethyl tert-butyl ether (ETBE), and tert-amyl methyl ether (TAME). Both a laboratory strain and natural isolates were able to degrade each compound after
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service