All Photos(1)
About This Item
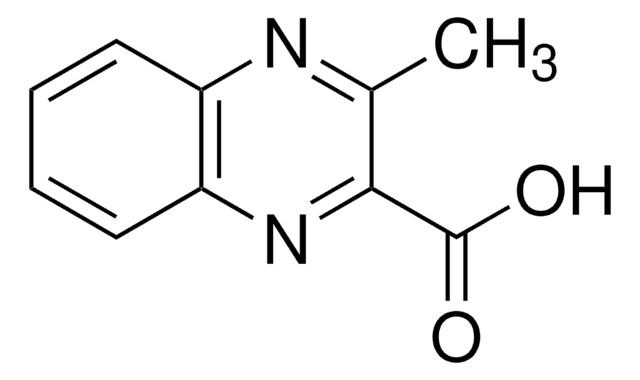
Empirical Formula (Hill Notation):
C9H6N2O2
CAS Number:
Molecular Weight:
174.16
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
208 °C (dec.) (lit.)
functional group
carboxylic acid
SMILES string
OC(=O)c1cnc2ccccc2n1
InChI
1S/C9H6N2O2/c12-9(13)8-5-10-6-3-1-2-4-7(6)11-8/h1-5H,(H,12,13)
InChI key
UPUZGXILYFKSGE-UHFFFAOYSA-N
General description
Linear and Freundlich adsorption isotherm coefficient of 2-quinoxalinecarboxylic acid has been evaluated.
Application
2-Quinoxalinecarboxylic acid has been used in the preparation of:
- N-(2-quinoxaloyl)-α-amino acids
- bisquinoxaloyl (bisquinoxalinecarbonyl) derivatives
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Joshua A Hagen et al.
Sensors (Basel, Switzerland), 11(7), 6645-6655 (2011-12-14)
Zinc oxide field effect transistors (ZnO-FET), covalently functionalized with single stranded DNA aptamers, provide a highly selective platform for label-free small molecule sensing. The nanostructured surface morphology of ZnO provides high sensitivity and room temperature deposition allows for a wide
Zhen-Juan Duan et al.
Analytical and bioanalytical chemistry, 401(7), 2291-2299 (2011-08-23)
A new molecularly imprinted polymer (MIP), selective for major metabolites of quinoxaline-1,4-dioxides was firstly prepared by combining surface molecular imprinting technique with the sol-gel process. Methyl-3-quinoxaline-2-carboxylic acid (MQCA) was used as template, 3-aminopropyltriethoxysilane as functional monomer, and tetraethoxysilicane as cross-linker.
M J Hutchinson et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 816(1-2), 15-20 (2005-01-25)
A method is described for the quantitative determination of quinoxaline-2-carboxylic acid (QCA) and methyl-3-quinoxaline-2-carboxylic acid (MQCA), the metabolites that have been designated as the marker residues for the veterinary drugs, carbadox and olaquindox, respectively, in swine tissue. The method is
Quinoxaline studies. 13. N-(2-Quinoxaloyl)-alpha-amino acids.
S Gerchakov et al.
Journal of medicinal chemistry, 9(2), 266-268 (1966-03-01)
M Rutalj et al.
Food additives and contaminants, 13(8), 879-882 (1996-11-01)
The concentration of quinoxaline-2-carboxylic acid (QCA) determined by HPLC after alkaline hydrolysis of liver and muscle of swine, ranged from < 3 ng/g to 45.3 ng/g in liver, and from < 3 ng/g to 10.8 ng/g in muscle samples. After
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service