136123
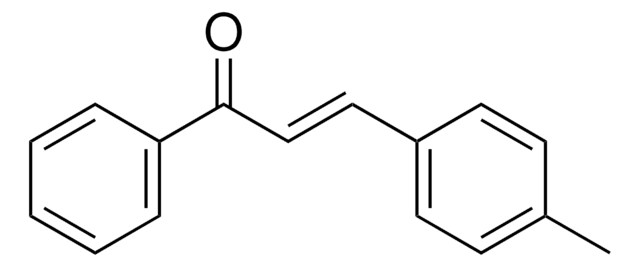
trans-Chalcone
97%
Synonym(s):
Benzylideneacetophenone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5CH=CHCOC6H5
CAS Number:
Molecular Weight:
208.26
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
bp
208 °C/25 mmHg (lit.)
mp
55-57 °C (lit.)
functional group
ketone
phenyl
SMILES string
[H]\C(=C(\[H])C(=O)c1ccccc1)c2ccccc2
InChI
1S/C15H12O/c16-15(14-9-5-2-6-10-14)12-11-13-7-3-1-4-8-13/h1-12H/b12-11+
InChI key
DQFBYFPFKXHELB-VAWYXSNFSA-N
Gene Information
human ... IL1B(3553)
rat ... Ar(24208)
Related Categories
General description
trans-Chalcone is an open chain flavonoid that may prevent lung and forestomach cancer.
Application
trans-Chalcone was used in the synthesis of cis and trans diphenyl cyclopropane. It was also used in screening of surface adsorbed species of isobutybenzene and 4-isobutylacetophenone on bulk fosfotungstic Wells-Dawson acid (H6P2W18O62.xH2O).
Biochem/physiol Actions
trans-Chalcone exhibits antifungal activity against Trichophyton rubrum. It is inhibitor of fatty acid synthase and α-amylase. It induces programmed cell death due to reduced mitochondrial transmembrane potential in Arabidopsis thaliana roots.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
P M Sivakumar et al.
Bioorganic & medicinal chemistry letters, 17(6), 1695-1700 (2007-02-06)
In order to develop relatively small molecules as antimycobacterial agents, twenty-five chalcones were synthesized, their activity was evaluated, and quantitative structure-activity relationship (QSAR) was developed. The synthesis was based on the Claisen-Schimdt scheme and the resultant compounds were tested for
Franco Chimenti et al.
Journal of medicinal chemistry, 52(9), 2818-2824 (2009-04-22)
A large series of substituted chalcones have been synthesized and tested in vitro for their ability to inhibit human monoamine oxidases A and B (hMAO-A and hMAO-B). While all the compounds showed hMAO-B selective activity in the micro- and nanomolar
Surface intermediate species of the 4-isobutylacetophenone adsorption-reaction over fosfotungstic Wells-Dawson heteropoly acid.
Matkovic SR, et al.
Latin American Applied Research, 39(2), 173-178 (2009)
Daniela Batovska et al.
European journal of medicinal chemistry, 44(5), 2211-2218 (2008-07-01)
A large series of chalcones were synthesized and studied against Staphylococcus aureus and Escherichia coli. Chalcones were either unsubstituted in ring A or possessed 4'-chloro or 3',4',5'-trimethoxy groups. Their other ring B was variously substituted. It was found that the
Wattenberg L.W., et al.
Cancer Letters, 15, 165-165 (1994)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service