All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H5ClN2S
CAS Number:
Molecular Weight:
184.65
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
mp
199-201 °C (lit.)
functional group
chloro
SMILES string
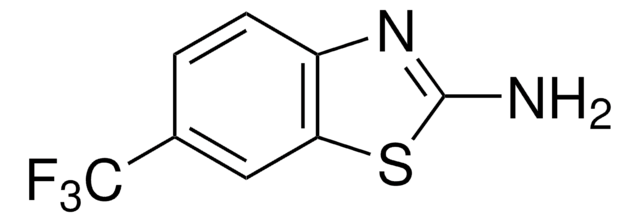
Nc1nc2ccc(Cl)cc2s1
InChI
1S/C7H5ClN2S/c8-4-1-2-5-6(3-4)11-7(9)10-5/h1-3H,(H2,9,10)
InChI key
VMNXKIDUTPOHPO-UHFFFAOYSA-N
General description
2-amino-6-chloro-benzothiazole on microwave irradiation in the presence of 1-butyl-3-methylimidazolium and inorganic anions yields fluorinated benzothiazolo[2,3-b]quinazoline-2H-ones analogues. It has synergistic effect on inhibitive performance of propargyl alcohol during corrosion of mild steel in boiling HCl solution.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synergistic effect of 2-amino-6-chloro-benzothiazole on inhibitive performance of propargyl alcohol during corrosion of mild steel in boiling hydrochloric acid solution.
Quraishi MA, et al.
Bulletin of Electrochemistry, 13(06), 257-259 (1997)
Jonathan R LaRochelle et al.
Bioorganic & medicinal chemistry, 25(24), 6479-6485 (2017-11-02)
The PTPN11 oncogene encodes the cytoplasmic protein tyrosine phosphatase SHP2, which, through its role in multiple signaling pathways, promotes the progression of hematological malignancies and other cancers. Here, we employ high-throughput screening to discover a lead chemical scaffold, the benzothiazolopyrimidones
Greener synthesis and photo-antiproliferative activity of novel fluorinated benzothiazolo [2, 3-b] quinazolines.
Arya K, et al. et al.
Medicinal Chemistry Research, 1-9 null
Ahmed S M Al-Janabi et al.
Journal of molecular structure, 1228, 129454-129454 (2020-10-27)
New Schiff bases {N'-(phenyl(pyridin-2-yl)methylene) isonicotinohydrazide (L1H), N1 -(naphthalen-1-yl)-N2 -(phenyl(pyridin-2-yl) methylidene) ethane-1,2-diamine (L2H), N-(6-chlorobenzo[d]thiazol-2-yl)-1-phenyl-1-(pyridin-2-yl) methanimine (L3H)}were synthesized by reaction of 2-benzoylpyridine with different amines (2-amino-6-chlorobenzothiazole, isonicotinohydrazide and N 1-(naphthalen-1-yl)ethane-1,2-diamine) and characterized by 1H-NMR, 13C-NMR, IR mass spectroscopy and elemental analysis. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service