All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H5NO
CAS Number:
Molecular Weight:
95.10
Beilstein:
105257
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
solid
bp
270 °C (lit.)
mp
62-67 °C (lit.)
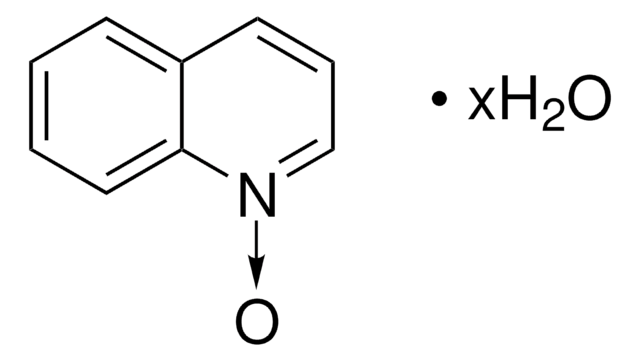
SMILES string
[O-][n+]1ccccc1
InChI
1S/C5H5NO/c7-6-4-2-1-3-5-6/h1-5H
InChI key
ILVXOBCQQYKLDS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Pyridine N-oxide axle with [2]rotaxanes was synthesized via an anion templated threading-followed-by-stoppering strategy.
Application
Pyridine N-oxide was used to study the FTIR spectra of pyridine N-oxide in acetonitrile.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
289.4 °F - closed cup
Flash Point(C)
143 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Takahiko Kojima et al.
Journal of the American Chemical Society, 133(44), 17901-17911 (2011-09-29)
Ruthenium(II)-acetonitrile complexes having η(3)-tris(2-pyridylmethyl)amine (TPA) with an uncoordinated pyridine ring and diimine such as 2,2'-bipyridine (bpy) and 2,2'-bipyrimidine (bpm), [Ru(II)(η(3)-TPA)(diimine)(CH(3)CN)](2+), reacted with m-chloroperbenzoic acid to afford corresponding Ru(II)-acetonitrile complexes having an uncoordinated pyridine-N-oxide arm, [Ru(II)(η(3)-TPA-O)(diimine)(CH(3)CN)](2+), with retention of the coordination
G Pitsevich et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 120, 585-594 (2014-01-01)
FTIR spectra of pyridine N-oxide and trichloroacetic acid H-bonded complex in acetonitrile were studied at 20 and 50°C. The calculations of equilibrium configurations of the complex and their IR spectra in harmonic- and anharmonic approximations were carried out at the
Highly efficient gold nanoparticle catalyzed deoxygenation of amides, sulfoxides, and pyridine N-oxides.
Yusuke Mikami et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(6), 1768-1772 (2011-01-29)
Jan Balzarini et al.
The Journal of antimicrobial chemotherapy, 55(2), 135-138 (2005-01-15)
Pyridine N-oxide derivatives represent a new class of anti-HIV compounds, for which some members exclusively act through inhibition of HIV-1 reverse transcriptase and thus characteristically behave as non-nucleoside reverse transcriptase inhibitors. Other members act, additionally or alternatively, at a post-integrational
Palladium-catalyzed (N-oxido-2-pyridinyl)methyl transfer from 2-(2-hydroxyalkyl)pyridine N-oxide to aryl halides by beta-carbon elimination.
Takafumi Suehiro et al.
Chemistry, an Asian journal, 4(8), 1217-1220 (2009-06-09)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service