109606
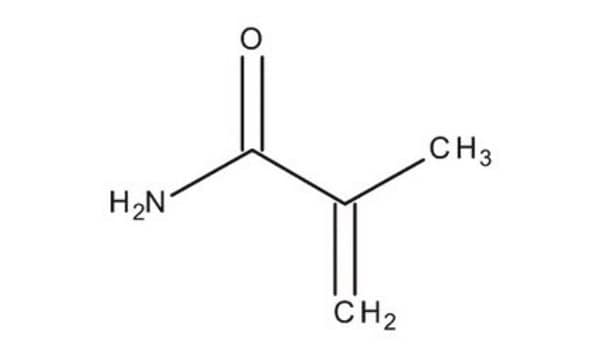
Methacrylamide
98%
Synonym(s):
2-Methylacrylamide, 2-Methylpropenamide, Methacrylic acid amide
About This Item
Recommended Products
Assay
98%
form
solid
mp
106-110 °C (lit.)
SMILES string
CC(=C)C(N)=O
InChI
1S/C4H7NO/c1-3(2)4(5)6/h1H2,2H3,(H2,5,6)
InChI key
FQPSGWSUVKBHSU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Synthesis of non-fouling poly brushes by photoinduced SET-LRP: This study highlights the use of photoinduced SET-LRP for the polymerization of methacrylamide, emphasizing its efficiency and the quality of the resulting polymers for non-fouling applications (M Vorobii, A de los Santos Pereira, 2015).
- Hydrolytic stability of methacrylamide and methacrylate in gelatin methacryloyl: The study investigates the hydrolytic stability of methacrylamide within gelatin methacryloyl, highlighting its stability and potential in biomedical applications (J Zheng, M Zhu, G Ferracci, NJ Cho, 2018).
- Two-step mechanisms of tumor selective delivery of N-(2-hydroxypropyl) methacrylamide copolymer conjugated with pirarubicin via an acid-cleavable linkage: This paper discusses the development of a copolymer conjugate for targeted cancer therapy, showcasing a two-step mechanism for enhanced drug delivery (H Nakamura, T Etrych, P Chytil, M Ohkubo, 2014).
- Backbone Degradable N-(2-Hydroxypropyl)methacrylamide Copolymer Conjugates with Gemcitabine and Paclitaxel: The research focuses on degradable copolymer conjugates for delivering cancer therapeutics, noting significant effects on tumor reduction and highlighting the impact of molecular weight (J Yang, R Zhang, H Pan, Y Li, Y Fang, 2017).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - STOT RE 2 - STOT SE 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service