All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H9N3
CAS Number:
Molecular Weight:
171.20
Beilstein:
127131
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
bp
222 °C/50 mmHg (lit.)
mp
90-92 °C (lit.)
SMILES string
N(c1ccccn1)c2ccccn2
InChI
1S/C10H9N3/c1-3-7-11-9(5-1)13-10-6-2-4-8-12-10/h1-8H,(H,11,12,13)
InChI key
HMMPCBAWTWYFLR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2,2′-Dipyridylamine (bipyam) can be used:
- As a bidentate N-donor ligand in the synthesis of various metal complexes.
- To synthesize Pd-polyoxovanadates, heterogeneous catalyst for the oxidation of benzylic hydrocarbons.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Manganese (II) complexes of the quinolone family member flumequine: Structure, antimicrobial activity and affinity for albumins and calf-thymus DNA
Barmpa A, et al.
Polyhedron, 145, 166-175 (2018)
Y Kobayashi et al.
Xenobiotica; the fate of foreign compounds in biological systems, 30(7), 683-692 (2000-08-30)
1. The effect of 2,2'-dipyridyl ketone and 2,2'-dipyridyl amine on the induction of hepatic microsomal cytochrome P450 (P450) and heme oxygenase was compared, and their effects on five different P450 isoforms (P4501A1, 3A2, 2B1, 2E1 and 2C11) in rat were
Runyu Tan et al.
Inorganic chemistry, 51(13), 7039-7049 (2012-06-13)
A novel multidentate ligand with 2,2'-dipyridylamine functionalities, 1,8-bis[4-(2,2'-dipyridylamino)phenylacetylenyl]anthracene (1), has been synthesized through a double Sonogashira coupling reaction and characterized by NMR spectroscopic, elemental, and X-ray diffraction analyses. Compound 1 can bind to either one metal center as a tetradentate
Heather R Lucas et al.
Journal of the American Chemical Society, 132(37), 12927-12940 (2010-08-24)
The kinetics, thermodynamics, and coordination dynamics are reported for O(2) and CO 1:1 binding to a series of pseudo-tetradentate ligand-copper(I) complexes ((D)LCu(I)) to give Cu(I)/O(2) and Cu(I)/CO product species. Members of the (D)LCu(I) series possess an identical tridentate core structure
Chao Tu et al.
Inorganic chemistry, 42(19), 5795-5797 (2003-09-16)
A novel hexadentate ligand, N,N,N',N',N",N"-hexa(2-pyridyl)-1,3,5-tris(aminomethyl)benzene (L), was designed and synthesized. The X-ray structure analysis reveals that the three dipyridylamine (DPA) groups of L are almost perpendicular to the central trimethylenebenzene, and two of them are spacially close to each other
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service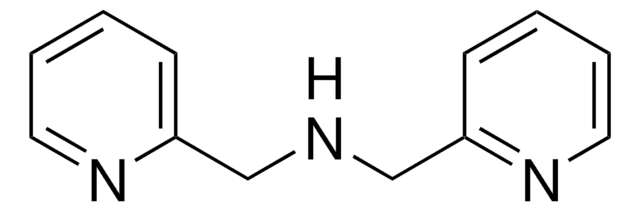












![2-[(Methylamino)methyl]pyridine 97%](/deepweb/assets/sigmaaldrich/product/structures/370/687/248fbc0c-2e59-447c-8191-2685dfb597d6/640/248fbc0c-2e59-447c-8191-2685dfb597d6.png)