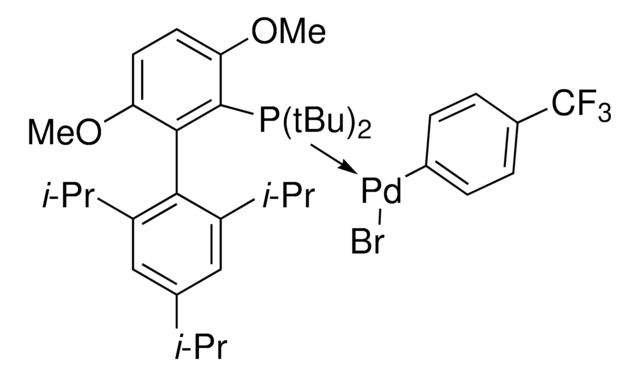
931853
SPhos Pd G6 acylation
≥95%
Synonym(s):
(SPhos)Pd(4-CH2CH2CONHSPh)Br
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C39H47BrNO6PPd
CAS Number:
Molecular Weight:
843.09
UNSPSC Code:
12352100
NACRES:
NA.21
Recommended Products
Quality Level
Assay
≥95%
form
powder
reaction suitability
reagent type: catalyst
reaction type: Cross Couplings
Application
SPhos Pd G6 acylation is an oxidative addition complex (OAC) of SPhos for use in bioconjugation. The pendant NHS-functional group allows coupling to protein amines through a selective acylation. The protein-OAC can then be reacted with a second, cysteine-containing protein. This facile approach allows for protein homodimerization as well as the formation of antibody-protein conjugates.
Learn more about G6 Buchwald precatalysts
Learn more about G6 Buchwald precatalysts
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Heemal H Dhanjee et al.
Journal of the American Chemical Society, 142(51), 21237-21242 (2020-12-16)
Palladium oxidative addition complexes (OACs) are traditionally accessed by treating an aryl halide-containing substrate with a palladium(0) source. Here, a new strategy to selectively prepare stable OACs from amino groups on native proteins is presented. The approach relies on an
Muhammad Jbara et al.
Angewandte Chemie (International ed. in English), 60(21), 12109-12115 (2021-03-18)
Organometallic reagents enable practical strategies for bioconjugation. Innovations in the design of water-soluble ligands and the enhancement of reaction rates have allowed for chemoselective cross-coupling reactions of peptides and proteins to be carried out in water. There are currently no
Ryan P King et al.
Organic letters, 23(20), 7927-7932 (2021-10-07)
The utilization of isolated Palladium Oxidative Addition Complexes (OACs) has had a significant impact on Pd-catalyzed and Pd-mediated cross-coupling reactions. Despite their importance, widespread utility of OACs has been limited by the instability of their precursor complexes. Herein, we report
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service