545953
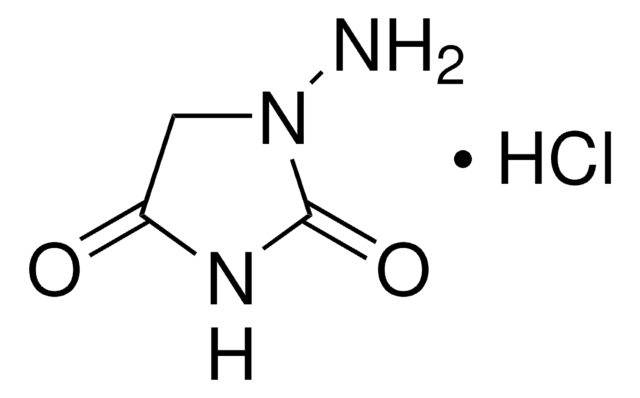
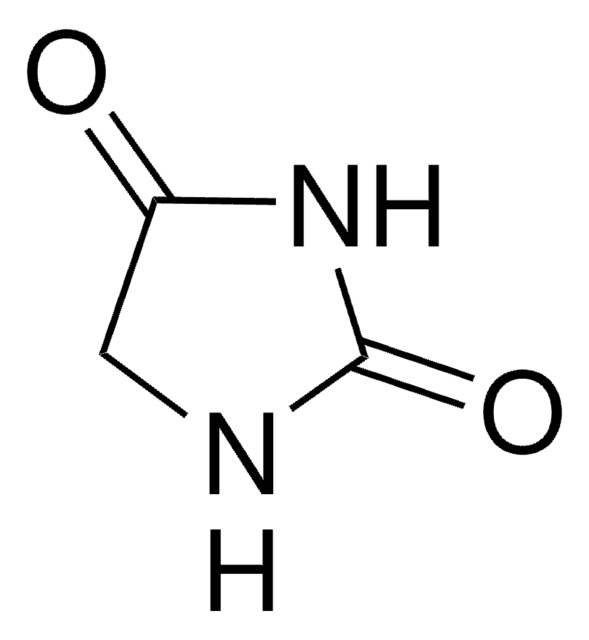
1-Aminohydantoin hydrochloride
98%
Synonym(s):
1-Amino-2,4-imidazolidinedione hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C3H5N3O2 · HCl
CAS Number:
Molecular Weight:
151.55
Beilstein:
3699376
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
201-205 °C (lit.)
SMILES string
Cl[H].NN1CC(=O)NC1=O
InChI
1S/C3H5N3O2.ClH/c4-6-1-2(7)5-3(6)8;/h1,4H2,(H,5,7,8);1H
InChI key
WEOHANUVLKERQI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
1-Aminohydantoin hydrochloride may be used as one of the reactants in the synthesis of (E)-1-(2-hydroxybenzylideneamino)imidazolidine-2,4-dione, dantrolene and dantrolene sodium.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of 1-Aminohydantoin Hydrochloride-(2-~(13) C,~(15) N_3) as Double Labeling Compound
Xu JF, et al.
Chemical World, 10, 015-015 (2012)
Crystal structure of (E)-1-(2-hydroxybenzylideneamino) imidazolidine-2, 4-dione, C10H9N3O3.
Hu, Lei, et al.
Zeitschrift fur Kristallographie, 230(2), 111-112 (2015)
Abdelselam Ali et al.
Bioorganic & medicinal chemistry letters, 20(2), 649-652 (2009-12-08)
A series of hydrazonotrifluorosulfonanilide derivatives were synthesized and evaluated for in vitro activity against the ectoparasites Ctenocephalides felis and Rhipicephalus sanguineus. Some compounds with excellent activity against tick were identified.
Takamitsu Hosoya et al.
Bioorganic & medicinal chemistry, 17(6), 2490-2496 (2009-02-24)
Suzuki-Miyaura coupling of 3-azido-5-(azidomethyl)phenylboronic acid pinacol ester with various aryl bromides affords corresponding diazido-functionalized biaryl compounds in good yields. This approach provides an easy access to radioisotope-free photoaffinity probes possessing biaryl structure. By using this method, we prepared a novel
Ascorbic Acid as an Initiator for the Direct C H Arylation of (Hetero) arenes with Anilines Nitrosated In Situ
Crisostomo, Fernando Pinacho, Tomas Martin, and Romen Carrillo
Angewandte Chemie (International Edition in English), 53.8 , 2181-2185 (2014)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service