413216
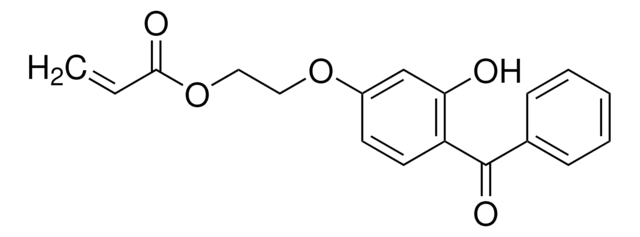
2-(4-Benzoyl-3-hydroxyphenoxy)ethyl acrylate
98%
Synonym(s):
2-Hydroxy-4-acryloxyethoxybenzophenone, 4-(2-Acryloxyethoxy)-2-hydroxybenzophenone
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
H2C=CHCO2CH2CH2OC6H3(OH)COC6H5
CAS Number:
Molecular Weight:
312.32
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
98%
form
powder
mp
77-80 °C (lit.)
SMILES string
Oc1cc(OCCOC(=O)C=C)ccc1C(=O)c2ccccc2
InChI
1S/C18H16O5/c1-2-17(20)23-11-10-22-14-8-9-15(16(19)12-14)18(21)13-6-4-3-5-7-13/h2-9,12,19H,1,10-11H2
InChI key
NMMXJQKTXREVGN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-(4-Benzoyl-3-hydroxyphenoxy)ethyl acrylate is a derivative of oxybenzone that is widely used as a UV-blocking agent in the fabrication of contact lenses. It contains both a vinyl group and an aromatic benzoyl group which makes it useful in polymerization and crosslinking reactions. This acrylate monomer is commonly used as a building block in the synthesis of polymers utilized in various coatings, adhesives, and dental fillings. Due to its unique structure, it can exhibit desirable properties such as high reactivity, good adhesion to a variety of substrates, and resistance to UV degradation.
Application
2-(4-Benzoyl-3-hydroxyphenoxy)ethylacrylate can be used as a monomer to synthesize fluorine-silicone acrylic resinto improve the UV resistance of the resin and make it more durable andlong-lasting.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
T V Chirila et al.
Journal of cataract and refractive surgery, 17(5), 596-603 (1991-09-01)
A tendency to reduce the use of benzophenone absorbers is currently evident in the manufacture of the UV-absorbing IOLs, mainly because the cutoff wavelengths are inferior to those provided by benzotriazoles. In principle, by incorporating large amounts of benzophenones it
T V Chirila et al.
Journal of cataract and refractive surgery, 15(5), 504-509 (1989-09-01)
A poly(2-hydroxyethyl methacrylate) hydrogel material exhibiting ultraviolet-absorbing properties was synthesized by simultaneous polymerization, crosslinking, and covalent bonding of an available polymerizable absorber. The two-stage leaching experiments carried out by aqueous extraction and the analysis of the concentrated extracts by high
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![2-[3-(2H-Benzotriazol-2-yl)-4-hydroxyphenyl]ethyl methacrylate 99%](/deepweb/assets/sigmaaldrich/product/structures/208/967/cf29567e-c125-41dc-b80a-66889fa1a679/640/cf29567e-c125-41dc-b80a-66889fa1a679.png)


![2,2′-Methylenebis[6-(2H-benzotriazol-2-yl)-4-(1,1,3,3-tetramethylbutyl)phenol] 99%](/deepweb/assets/sigmaaldrich/product/structures/236/824/ce89085c-b9e1-4ea0-8157-44b6f9466ed6/640/ce89085c-b9e1-4ea0-8157-44b6f9466ed6.png)