SAB5703041
Anti-Pan-Akt Antibody, clone 3K3H0, Rabbit Monoclonal
About This Item
Recommended Products
biological source
rabbit
Quality Level
material
colorless
clone
3K3H0, monoclonal
form
liquid
mol wt
60 kDa
species reactivity
rat, rat, human
concentration
1 mg/mL
technique(s)
immunofluorescence: 1:50 - 1:200
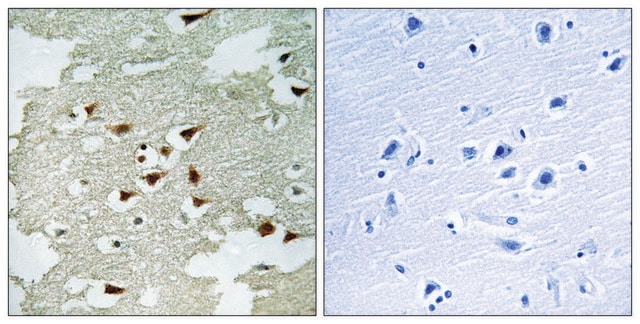
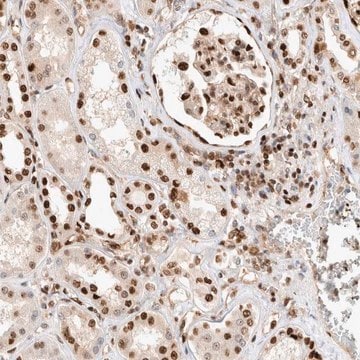
immunohistochemistry: 1:50 - 1:200
immunoprecipitation (IP): 1:50 - 1:200
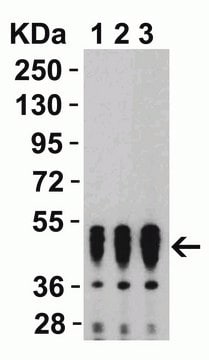
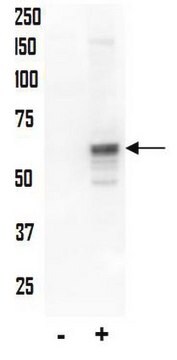
western blot: 1:1000 - 1:2000
color
colorless
isotype
IgG
immunogen sequence
MSDVAIVKEGWLHKRGEYIKTWRPRYFLLKNDGTFIGYKERPQDVDQREAPLNNFSVAQCQLMKTERPRPNTFIIRCLQWTTVIERTFHVETPEEREEWTTAIQTVADGLKKQEEEEMDFRSG
shipped in
wet ice
storage temp.
−20°C
target post-translational modification
unmodified
Gene Information
human ... AKT(207)
General description
Immunogen
Application
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2 - Skin Sens. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Choose from one of the most recent versions:
Certificates of Analysis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documents section.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service