I8132
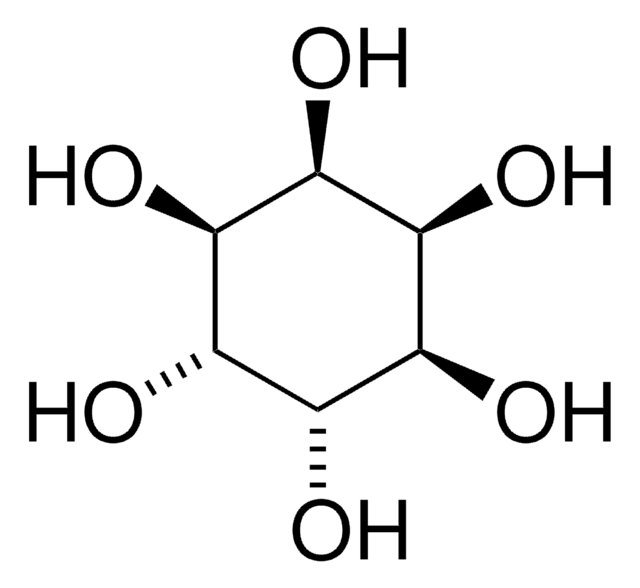
scyllo-Inositol
≥98%
Synonym(s):
1,3,5/2,4,6-Hexahydroxycyclohexane, DTLET
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C6H12O6
CAS Number:
Molecular Weight:
180.16
MDL number:
UNSPSC Code:
12352207
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Assay
≥98%
SMILES string
O[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@H](O)[C@H]1O
InChI
1S/C6H12O6/c7-1-2(8)4(10)6(12)5(11)3(1)9/h1-12H/t1-,2-,3+,4+,5-,6-
InChI key
CDAISMWEOUEBRE-CDRYSYESSA-N
Other Notes
Naturally occurring isomer of myo-inositol.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Christophe Michon et al.
Communications biology, 3(1), 93-93 (2020-03-04)
A rare stereoisomer of inositol, scyllo-inositol, is a therapeutic agent that has shown potential efficacy in preventing Alzheimer's disease. Mycobacterium tuberculosis ino1 encoding myo-inositol-1-phosphate (MI1P) synthase (MI1PS) was introduced into Bacillus subtilis to convert glucose-6-phosphate (G6P) into MI1P. We found that
Aaron Y Lai et al.
Biochimica et biophysica acta, 1822(10), 1629-1637 (2012-07-18)
scyllo-Inositol (SI) is an endogenous inositol stereoisomer known to inhibit aggregation and fibril formation of the amyloid-beta peptide (Aβ). Human clinical trials using SI to treat Alzheimer disease (AD) patients have shown potential benefits. In light of the growing therapeutic
Kevin A Dasilva et al.
Experimental neurology, 223(2), 311-321 (2009-09-12)
Structural insight into the conformational changes associated with aggregation and assembly of fibrils has provided a number of targets for therapeutic intervention. Solid-state NMR, hydrogen/deuterium exchange and mutagenesis strategies have been used to probe the secondary and tertiary structure of
Yedi Sun et al.
Bioorganic & medicinal chemistry, 16(15), 7177-7184 (2008-07-19)
scyllo-Inositol has shown promise as a potential therapeutic for Alzheimer's disease, by directly interacting with the amyloid beta (Abeta) peptide to inhibit Abeta42 fiber formation. To explore the molecular details of the inositol-Abeta42 interaction, a series of scyllo-inositol derivatives have
Sara Alam et al.
Current biology : CB, 33(11), 2175-2186 (2023-05-11)
Most eukaryotes respire oxygen, using it to generate biomass and energy. However, a few organisms have lost the capacity to respire. Understanding how they manage biomass and energy production may illuminate the critical points at which respiration feeds into central
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service