All Photos(1)
About This Item
Empirical Formula (Hill Notation):
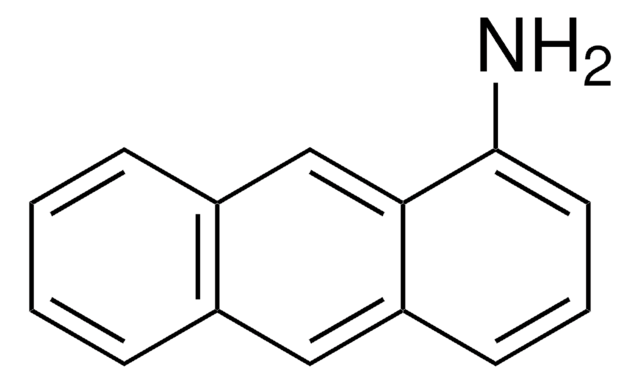
C18H13N
CAS Number:
Molecular Weight:
243.30
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
solid
mp
209-211 °C (lit.)
SMILES string
Nc1cc2c3ccccc3ccc2c4ccccc14
InChI
1S/C18H13N/c19-18-11-17-13-6-2-1-5-12(13)9-10-15(17)14-7-3-4-8-16(14)18/h1-11H,19H2
InChI key
KIVUHCNVDWYUNP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Produces tumors in mice.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
K B Delclos et al.
Cancer research, 47(23), 6272-6277 (1987-12-01)
6-Nitrochrysene (NC) and 6-aminochrysene (AC) have been shown to be potent lung and liver carcinogens when administered in multiple i.p. doses to preweanling mice. 1,6-Dinitropyrene has been shown to be a strong hepatocarcinogen but a weak lung carcinogen in this
F K Friedman et al.
Pharmacology, 31(4), 194-202 (1985-01-01)
The microsomal cytochrome P-450-dependent aryl hydrocarbon hydroxylase is important in the detoxification of polycyclic hydrocarbons as well as their activation to cytotoxic or carcinogenic derivatives. We have studied compounds that can modify the activity of this enzyme system. Three types
S Lahmy et al.
Toxicology, 29(4), 345-356 (1984-02-01)
By microspectrofluorimetry on single living cells (murine fibroblasts 3T3), we have obtained monoexponential decreases of fluorescence intensity for benzo[a]pyrene (B[a]P) and 6-aminochrysene (6a-chrysene) metabolism. These kinetics are characteristics of B[a]P and 6a-chrysene metabolism and histograms can be drawn from the
T Marczylo et al.
Mutagenesis, 9(3), 233-239 (1994-05-01)
6-Aminochrysene was converted into mutagen(s), in the Ames test in the presence of Aroclor 1254-induced hepatic S9, microsomal and cytosolic fractions, the first being the least and the last the most efficient activation system. The cytosolic activation of 6-aminochrysene decreased
S M Morris et al.
Mutation research, 310(1), 45-54 (1994-10-01)
Cells from the human lymphoblastoid cell line, AHH-1, were exposed to two direct-acting mutagens, ethyl methanesulfonate (EMS) and ethyl nitrosourea (ENU), and to three carcinogens that require metabolic activation to an electrophile, benzo[a]pyrene (B(a)P), 6-aminochrysene (6-AC), and 6-nitrochrysene (6-NC); mutation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service