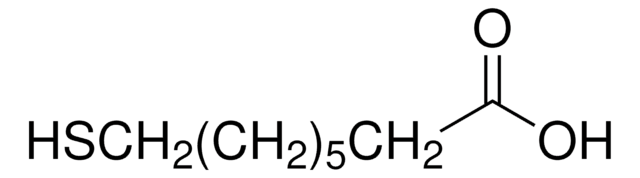745774
8-Amino-1-octanethiol hydrochloride
95%
Synonym(s):
8-Mercaptooctylamine hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H19NS · HCl
CAS Number:
Molecular Weight:
197.77
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
95%
form
solid
mp
158-163 °C
storage temp.
2-8°C
SMILES string
SCCCCCCCCN.Cl
InChI
1S/C8H19NS.ClH/c9-7-5-3-1-2-4-6-8-10;/h10H,1-9H2;1H
InChI key
CCWZBRKMJBSNQS-UHFFFAOYSA-N
Related Categories
General description
8-Amino-1-octanethiol hydrochloride (8-AOT) is an amine based alkanethiol that can be used as a self-assembled monolayer (SAM) on a variety of surfaces. It facilitates the immobilization of surface atoms and controls the physiochemical properties of the substrate.
Application
8-AOT can be used in the surface functionalization of gold electrodes for the trace analysis of oligonucleotide using poly nucleic acid (PNA) based ion-channel sensors. It can be used in the fabrication of peptide microarray for surface plasmon resonance based screening of protein kinase activity.
Self Assembly Molecules (SAMs); the amino group is usually modified using amine-reactive materials; such as proteins or biomaterials; to functionalize the gold surface. It is used for biosenors with large amounts of immobilized protein and suppression of nonspecific adsorption
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jolene L Lau et al.
ACS nano, 5(10), 7722-7729 (2011-09-09)
A high-affinity RNA aptamer (K(d) = 50 nM) was efficiently identified by SELEX against a heteroaryldihydropyrimidine structure, chosen as a representative drug-like molecule with no cross reactivity with mammalian or bacterial cells. This aptamer, its weaker-binding variants, and a known
Tillmann Utesch et al.
Langmuir : the ACS journal of surfaces and colloids, 28(13), 5761-5769 (2012-03-06)
Sulfite oxidase (SO) is an enzyme catalyzing the terminal step of the metabolism of sulfur-containing amino acids that is essential for almost all living organisms. The catalytic activity of SO in vertebrates strongly depends on the efficiency of the intramolecular
Trace analysis of an oligonucleotide with a specific sequence using PNA-based ion-channel sensors
Aoki H and Umezawa Y
Analyst, 128(6), 681-685 (2003)
Evaluation of protein kinase activities of cell lysates using peptide microarrays based on surface plasmon resonance imaging
Mori T, et al.
Analytical Biochemistry, 375(2), 223-231 (2008)
Photooxidation of amine-terminated self-assembled monolayers on gold
Lee S, et al.
The Journal of Physical Chemistry C, 114(23), 10512-10519 (2010)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service