All Photos(1)
About This Item
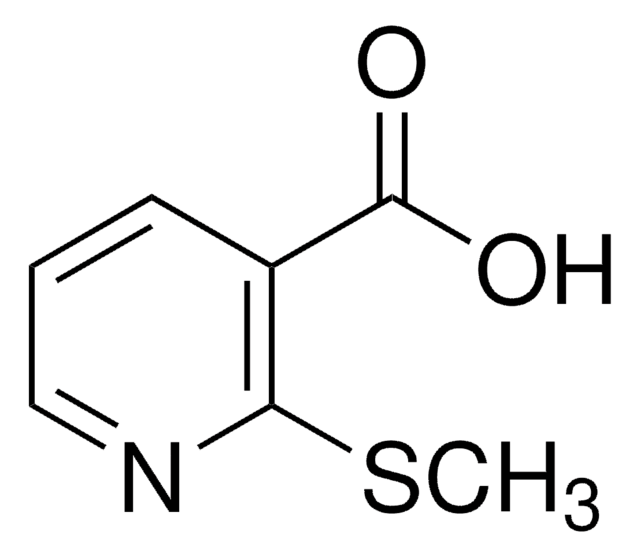
Empirical Formula (Hill Notation):
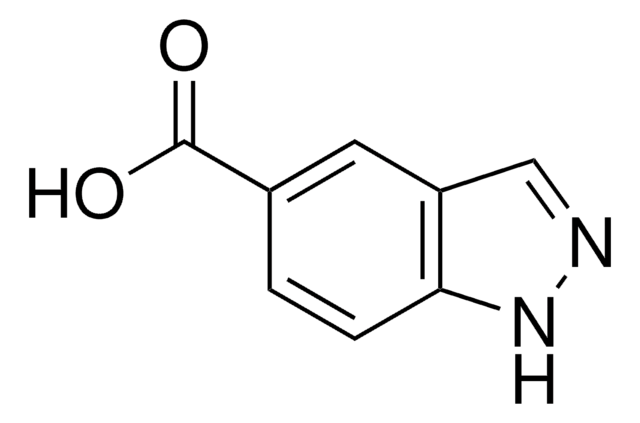
C8H6N2O2
CAS Number:
Molecular Weight:
162.15
Beilstein:
6354
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥97.0% (HPLC)
form
powder
color
beige
mp
266-270 °C (dec.)
SMILES string
OC(=O)c1n[nH]c2ccccc12
InChI
1S/C8H6N2O2/c11-8(12)7-5-3-1-2-4-6(5)9-10-7/h1-4H,(H,9,10)(H,11,12)
InChI key
BHXVYTQDWMQVBI-UHFFFAOYSA-N
Application
Indazole-3-carboxylic acid may be used in the synthesis of the following:
- N-(8-methyl-azabicyclo[3.2.11]oct-3-yl)-1H-indazole-3-carboxamide
- N-(1- benzyl-4-methylhexahydro-1H-1,4-diazepin-6-yl)-1H-indazole-3-carboxamide
- 1H-indazole-3-carboxamide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Per Ole M Gundersen et al.
Drug testing and analysis, 11(1), 51-67 (2018-07-12)
Synthetic cannabinoids are one of the most significant groups within the category new psychoactive substances (NPS) and in recent years new compounds have continuously been introduced to the market of recreational drugs. A sensitive and quantitative screening method in urine
"Synthesis and biochemical evaluation of tritium-labeled 1-methyl-N-(8-methyl-8-azabicyclo[3.2.1]oct-3-yl)-1H-indazole-3-carboxamide,a useful radioligand for 5HT3 receptors"
Robertson.WD, et al.
Journal of Medicinal Chemistry, 33(12), 3176-3181 (1990)
P Khanna et al.
Biochemistry, 40(5), 1441-1450 (2001-02-15)
N-Methyltryptophan oxidase (MTOX) is a flavoenzyme that catalyzes the oxidative demethylation of N-methyl-L-tryptophan and other N-methyl amino acids, including sarcosine, which is a poor substrate. The Escherichia coli gene encoding MTOX (solA) was isolated on the basis of its sequence
"Convenient Synthesis of N-(2, 2-Dimethyl-1, 3-dioxan-5-yl)-1 H-indazole-3-carboxamide, the Intermediate of 5-HT3 Receptor Antagonist"
Morie T, et al.
Synthetic Communications, 27(4), 559-566 (1997)
Basic and applied research in the study of indazole carboxylic acids.
B Silvestrini
Chemotherapy, 27 Suppl 2, 9-20 (1981-01-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service