All Photos(3)
About This Item
Empirical Formula (Hill Notation):
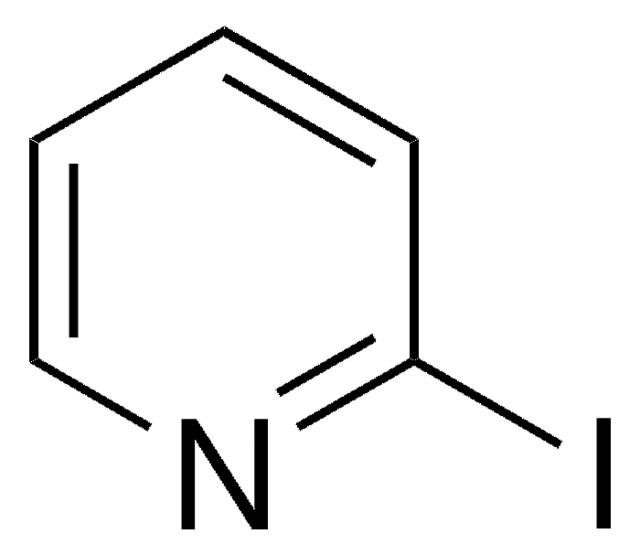
C5H4IN
CAS Number:
Molecular Weight:
205.00
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
53-56 °C (lit.)
SMILES string
Ic1cccnc1
InChI
1S/C5H4IN/c6-5-2-1-3-7-4-5/h1-4H
InChI key
XDELKSRGBLWMBA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
3-Iodopyridine is a heteroaryl halide. It undergoes microwave-assisted coupling with heterocyclic compounds (pyrazole, imidazole, pyrrole and indole) to afford the corresponding N-3-pyridinyl-substituted heterocyclic compounds.
Application
3-Iodopyridine may be used to synthesize following pyridine alkaloids:
- theonelladins C
- theonelladins D
- niphatesine C
- xestamine D
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
224.1 °F - closed cup
Flash Point(C)
106.7 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Microwave-assisted solvent-and ligand-free copper-catalysed cross-coupling between halopyridines and nitrogen nucleophiles.
Ernst, R. F.
Green Chemistry, 13(1), 42-45 (2011)
Alina K Feldman et al.
Organic letters, 6(22), 3897-3899 (2004-10-22)
[reaction: see text] 1,4-Disubstituted 1,2,3-triazoles are obtained in excellent yields by a convenient one-pot procedure from a variety of readily available aromatic and aliphatic halides without isolation of potentially unstable organic azide intermediates.
Synthesis of pyridine alkaloids via Pd-catalyzed coupling of 3-iodopyridine, 1, ?-dienes and nitrogen nucleophiles.
Larock RC and Wang Y.
Tetrahedron Letters, 43(1), 21-23 (2002)
Cameron C Bright et al.
Physical chemistry chemical physics : PCCP, 19(46), 31072-31084 (2017-11-21)
Small nitrogen containing heteroaromatics are fundamental building blocks for many biological molecules, including the DNA nucleotides. Pyridine, as a prototypical N-heteroaromatic, has been implicated in the chemical evolution of many extraterrestrial environments, including the atmosphere of Titan. This paper reports
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service