532851
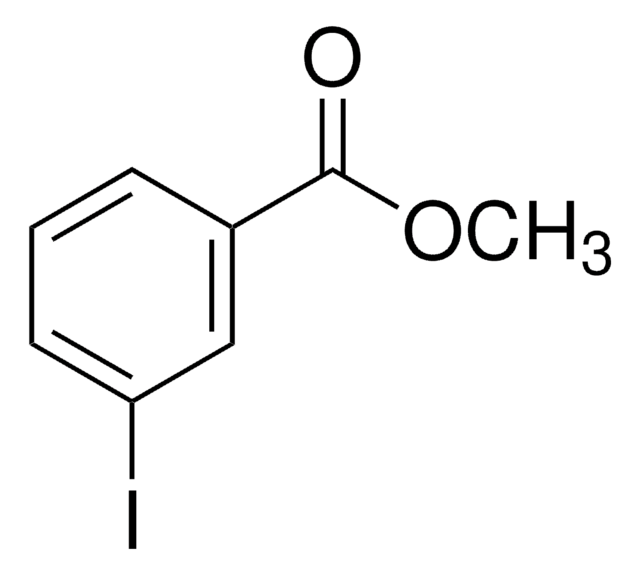
Methyl 2-iodobenzoate
97%
Synonym(s):
Methyl o-iodobenzoate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
IC6H4CO2CH3
CAS Number:
Molecular Weight:
262.04
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.604 (lit.)
bp
149-150 °C/10 mmHg (lit.)
density
1.784 g/mL at 25 °C (lit.)
SMILES string
COC(=O)c1ccccc1I
InChI
1S/C8H7IO2/c1-11-8(10)6-4-2-3-5-7(6)9/h2-5H,1H3
InChI key
BXXLTVBTDZXPTN-UHFFFAOYSA-N
Related Categories
General description
Methyl 2-iodobenzoate can be prepared from 2-iodobenzoic acid via esterification. It undergoes cobalt-catalyzed cyclization with aldehydes to form phthalide derivatives. The microbial dihydroxylation of methyl 2-iodobenzoate forms a nonracemic iodocyclohexene carboxylate intermediate. This intermediate forms the precursor for preparing kibdelone C.
Application
Methyl 2-iodobenzoate may be used in the preparation of:
- N-substituted 4-methylene-3,4-dihydro-1(2H)-isoquinolin-1-ones
- methyl diphenylacetylene-2-carboxylate
- methyl 2-heptynylphenylbenzoate
- (E)-2-[3-[3-[2-(7-chloro-2-quinolinyl)ethenyl]phenyl]-3-oxopropyl]benzoic acid methyl ester
- 4-(2-carbomethoxyphenyl)-3-(1-methylethoxy)cyclobut-3-ene-1,2-dione
- 3-(2-carbomethoxyphenyl)-4-methylcyclobuten-3-ene-1,2-dione 2-(ethylene acetal)
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Palladium homogeneous and supported catalysis: synthesis of functional acetylenics and cyclisation to heterocycles.
Villemin D and Goussu D.
Heterocycles, 29(7), 1255-1261 (1989)
Highly Efficient Cyclization of o?Iodobenzoates with Aldehydes Catalyzed by Cobalt Bidentate Phosphine Complexes: A Novel Entry to Chiral Phthalides.
Chang HT, et al.
Chemistry (Weinheim An Der Bergstrasse, Germany), 13(15), 4356-4363 (2007)
3-Stannylcyclobutenediones as nucleophilic cyclobutenedione equivalents. Synthesis of substituted cyclobutenediones and cyclobutenedione monoacetals and the beneficial effect of catalytic copper iodide on the Stille reaction.
Liebeskind LS and Fengl RW.
The Journal of Organic Chemistry, 55(19), 5359-5364 (1990)
An efficient synthesis of LTD4 antagonist L-699,392.
The Journal of Organic Chemistry, 58(14), 3731-3735 (1993)
Synthesis of Polyimides via the Palladium-Catalyzed Carbonylation of Bis (o-iodo esters) and Diamines.
Perry RJ, et al.
Macromolecules, 28(10), 3509-3515 (1995)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service