All Photos(1)
About This Item
Empirical Formula (Hill Notation):
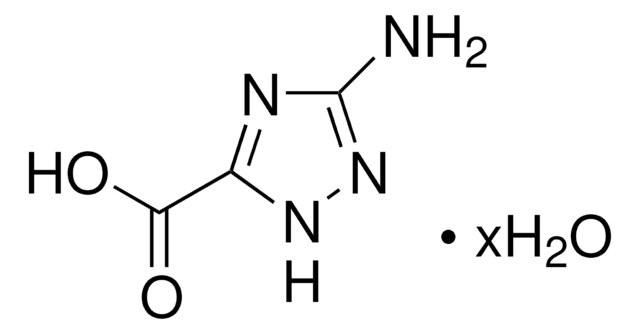
C11H12N2O3
CAS Number:
Molecular Weight:
220.22
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
194-195 °C (lit.)
SMILES string
CN1[C@@H]([C@H](CC1=O)C(O)=O)c2cccnc2
InChI
1S/C11H12N2O3/c1-13-9(14)5-8(11(15)16)10(13)7-3-2-4-12-6-7/h2-4,6,8,10H,5H2,1H3,(H,15,16)/t8-,10+/m0/s1
InChI key
DEYLVDCFTICBTB-WCBMZHEXSA-N
Related Categories
General description
Carboxyl group of trans-4-cotininecarboxylic acid (carboxycotinine) can be used for chemical crosslinking.
Application
trans-4-Cotininecarboxylic acid was used in the preparation of cotinine-conjugated horseradish peroxidase during immunoblot analysis. Anion of trans-4-cotininecarboxylic acid has been employed as pyridyl-carboxylate ligand in the preparation of polymeric copper(II) complex.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
The use of trans-4-cotininecarboxylate to construct a polymeric copper (II) azido complex: Hydro (solvo) thermal synthesis, structure and magnetic properties.
Luo F, et al.
Inorgorganica Chimica Acta, 363(14), 4117-4122 (2010)
Hyori Kim et al.
Experimental & molecular medicine, 45, e43-e43 (2013-09-28)
We present a bispecific antibody that recognizes an antigen and a hapten and can be applied to various biological assays, including immunoblotting and immunoprecipitation. In immunoblot analysis of serum, an anti-C5 × anti-cotinine bispecific tandem single-chain variable fragment (scFv)-Fc fusion
Soomin Yoon et al.
Journal of cancer research and clinical oncology, 140(2), 227-233 (2013-12-03)
Cotinine has optimal characteristics as a hapten for pre-targeted radioimmunotherapy (PRIT). This study was performed to evaluate the applicability of cotinine/anti-cotinine antibody to PRIT. We developed and prepared a tandem, single-chain, variable fragment Fc fusion protein [tandem single-chain variable fragment
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service