276316
1-Bromo-3,5-dimethylbenzene
97%
Synonym(s):
5-Bromo-m-xylene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
(CH3)2C6H3Br
CAS Number:
Molecular Weight:
185.06
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
refractive index
n20/D 1.549 (lit.)
bp
202-204 °C (lit.)
density
1.362 g/mL at 25 °C (lit.)
SMILES string
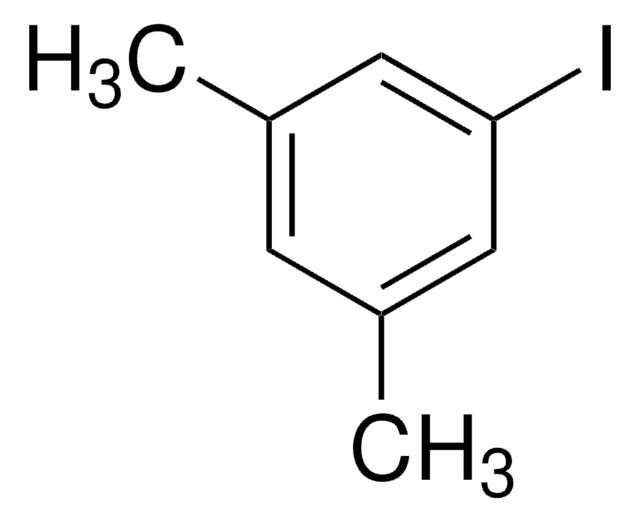
Cc1cc(C)cc(Br)c1
InChI
1S/C8H9Br/c1-6-3-7(2)5-8(9)4-6/h3-5H,1-2H3
InChI key
LMFRTSBQRLSJHC-UHFFFAOYSA-N
Related Categories
General description
Palladium catalyzed carbon-oxygen coupling of 1-bromo-3,5-dimethylbenzene and o-cresol to potassium hydroxide to produce o-tolyl-3,5-xylyl ether has been reported.
Application
1-Bromo-3,5-dimethylbenzene has been used in the synthesis of substituted 2,2′-bis(diphenylphosphanylmethyl)-1,1′-binaphthyl derivatives.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
188.6 °F - closed cup
Flash Point(C)
87 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Highly Selective Catalyst Systems for the Hydroformylation of Internal Olefins to Linear Aldehydes This work was supported by Oxeno Olefinchemie GmbH and the State of Mecklenburg-West Pommerania. Dr. C. Fischer and Mrs. S. Buchholz are thanked for their excellent technical support.
Holger Klein et al.
Angewandte Chemie (International ed. in English), 40(18), 3408-3411 (2001-10-10)
Vittoria M Blasucci et al.
The journal of physical chemistry. A, 114(11), 3932-3938 (2010-03-20)
Tunable solvent systems couple homogeneous catalytic reactions to heterogeneous separations, thereby combining multiple unit operations into a single step and subsequently reducing waste generation and improving process economics. In addition, tunable solvents can require less energy than traditional separations, such
Tapan K Pal et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 21(52), 19064-19070 (2015-11-21)
By using a bent tetracarboxylic acid ligand that incorporates a pendent-NH2 functional group, a 3D Zn(II)-framework (1) based on Zn2 (CO2)4 secondary building units and Zn12 (CO2)24 supramolecular building blocks has been synthesized. Framework 1 is thermally less stable, which
Asami Yoshii et al.
Chemistry, an Asian journal, 15(14), 2181-2186 (2020-05-26)
Oligo-meta-phenylenes have been designed and synthesized as multipotent base materials of single-layer organic light-emitting devices. Simple molecular structures of oligo-meta-phenylenes composed of linear phenylene arrays benefited from the wealth of modern reactions available for biaryl couplings and were concisely synthesized
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service