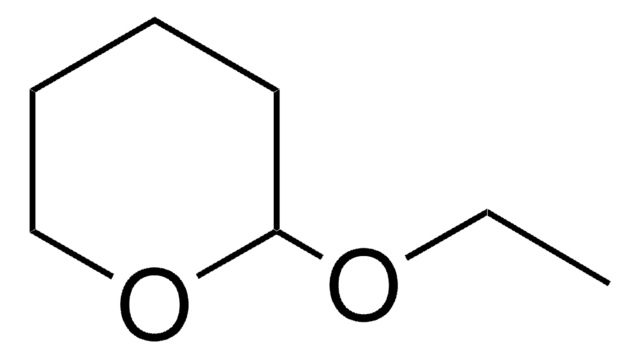
189170
5,6-Dihydro-4-methoxy-2H-pyran
95%
Synonym(s):
4-Methoxy-5,6-dihydropyran
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H10O2
CAS Number:
Molecular Weight:
114.14
Beilstein:
1341205
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
liquid
refractive index
n20/D 1.462 (lit.)
bp
59 °C/20 mmHg (lit.)
density
1.022 g/mL at 25 °C (lit.)
functional group
ether
SMILES string
COC1=CCOCC1
InChI
1S/C6H10O2/c1-7-6-2-4-8-5-3-6/h2H,3-5H2,1H3
InChI key
FSMHNRHLQAABPS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
5,6-Dihydro-4-methoxy-2H-pyran is suitable reagent for protection of nucleoside hydroxyl functions.
Application
5,6-Dihydro-4-methoxy-2H-pyran was used in solid-phase synthesis of series of oligoribonucleotides. It was also used as reagent for the protection of chiral alcohols.
accessory
Product No.
Description
Pricing
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
114.8 °F - closed cup
Flash Point(C)
46 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Tetrahedron Letters, 33, 2371-2371 (1992)
A J Leigh et al.
Biochemistry, 31(37), 8978-8983 (1992-09-22)
The substrate stereospecificity of phosphatidylinositol-specific phospholipase C from Bacillus cereus is examined using the resolved optical isomers of synthetic myo-inositol 1-(4-nitrophenyl phosphate), a chromogenic substrate for the phospholipase. The synthetic route employs mild acid-labile protecting groups and separation of the
1, 1-Diethoxybut-2-ene as a Precursor of (2-Hydroxyethyl)-Substituted Alkoxy Dienes: Convenient Intermediates for a New Synthesis of 2-Substituted and 2, 6-Disubstituted Tetrahydro-4H-pyran-4-ones.
Prandi C and Venturello P.
The Journal of Organic Chemistry, 59(12), 3494-3496 (1994)
C Lehmann et al.
Nucleic acids research, 17(7), 2379-2390 (1989-04-11)
Efficient solid-phase synthesis of a series of oligoribonucleotides of up to 20 residues is described that utilises the 9-fluorenylmethoxycarbonyl group (Fmoc) for 5'-protection and 4-methoxytetrahydropyran-4-yl (Mthp) for 2'-protection of ribonucleotide monomers and a phosphoramidite coupling procedure. The Fmoc group is
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service