All Photos(1)
About This Item
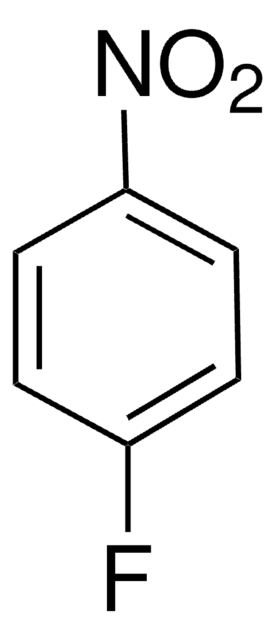
Linear Formula:
FC6H4OCH3
CAS Number:
Molecular Weight:
126.13
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
liquid
refractive index
n20/D 1.488 (lit.)
bp
158 °C/743 mmHg (lit.)
density
1.104 g/mL at 25 °C (lit.)
SMILES string
COc1cccc(F)c1
InChI
1S/C7H7FO/c1-9-7-4-2-3-6(8)5-7/h2-5H,1H3
InChI key
MFJNOXOAIFNSBX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3-Fluoroanisole was used in the synthesis of 4-fluoro-5,6-dihydroxytryptamine. It was also used in the synthesis of 3-fluoro- and 5-fluoronoradrenaline.
Signal Word
Warning
Hazard Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
111.2 °F - closed cup
Flash Point(C)
44 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
M Kawase et al.
Journal of medicinal chemistry, 33(8), 2204-2211 (1990-08-01)
The 5,6-dihydroxytryptamine (5,6-DHT) derivatives 4-fluoro- and 7-fluoro-5,6-DHTs (26a,b) and 4,7-difluoro-5,6-DHT (26c) were synthesized from 3-fluoroanisole (1) and 1,4-difluoro-2,3-dimethoxybenzene (13), respectively. Efficient methods were developed for the conversion of 1 to 4-fluoro- and 7-fluoro-5,6-bis(benzyloxy)indoles (12a,b, respectively), and 13 to 4,7-difluoro-5,6-[( diphenylmethylene)dioxy]indole
Synthesis and EPR investigations of fluorocatecholamines.
Stegmann HB, et al.
Journal of the Chemical Society. Perkin Transactions 1, 2(3), 547-555 (1994)
Oriol Planas et al.
Science (New York, N.Y.), 367(6475), 313-317 (2020-01-18)
Bismuth catalysis has traditionally relied on the Lewis acidic properties of the element in a fixed oxidation state. In this paper, we report a series of bismuth complexes that can undergo oxidative addition, reductive elimination, and transmetallation in a manner
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service