All Photos(2)
About This Item
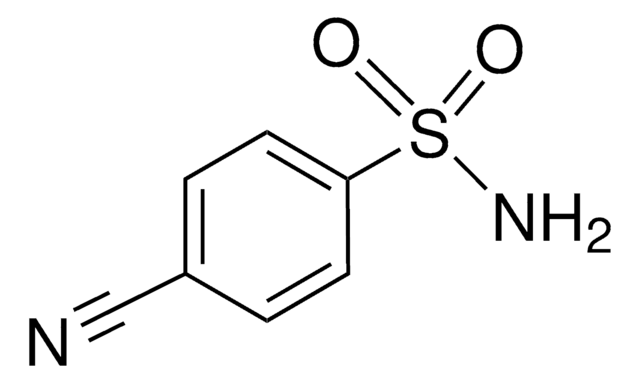
Linear Formula:
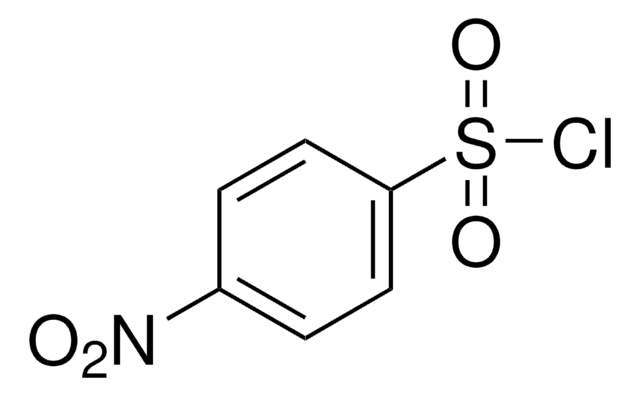
O2NC6H4SO2NH2
CAS Number:
Molecular Weight:
202.19
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
178-180 °C (lit.)
SMILES string
NS(=O)(=O)c1ccc(cc1)[N+]([O-])=O
InChI
1S/C6H6N2O4S/c7-13(11,12)6-3-1-5(2-4-6)8(9)10/h1-4H,(H2,7,11,12)
InChI key
QWKKYJLAUWFPDB-UHFFFAOYSA-N
General description
4-Nitrobenzenesulfonamide is the nitrene source during on pot procedure for copper(I)-catalyzed asymmetric alkene aziridination. It reacts with diazacrown ether, N,N′-dibenzyl-1,7,10,16-tetraoxo-4,13-diazacyclooctadecane to form molecular complexes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The 1: 2 and 1: 1 molecular complexes of N,N'-dibenzyl-4, 13-diaza-18-crown-6 with 4-nitrobenzenesulfonamide and dithiooxamide.
Fonari MS, et al.
Journal of Molecular Structure, 794(1), 110-114 (2006)
Copper (I)-catalyzed asymmetric alkene aziridination mediated by PhI (OAc)< sub> 2</sub>: a facile one-pot procedure.
Kwong HL, et al.
Tetrahedron Letters, 45(20), 3965-3968 (2004)
Jonathan T Park et al.
Chembiochem : a European journal of chemical biology, 16(5), 811-818 (2015-02-24)
Nitroreductases (NRs) and ene-reductases (ERs) both utilize flavin mononucleotide cofactors but catalyze distinct reactions. NRs reduce nitroaromatics, whereas ERs reduce unsaturated C=C double bonds, and these functionalities are known to somewhat overlap. Recent studies on the ER xenobiotic reductase A
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service