06372
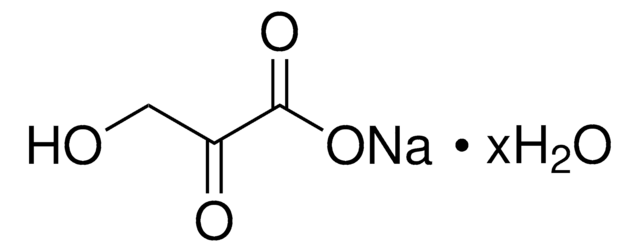
β-Hydroxypyruvic acid
≥95.0% (dry substance, T)
Synonym(s):
3-Hydroxy-2-oxopropanoic acid
About This Item
Recommended Products
Quality Level
Assay
≥95.0% (dry substance, T)
form
powder and chunks
impurities
≤15.0% water
storage temp.
2-8°C
SMILES string
OC(C(CO)=O)=O
InChI
1S/C3H4O4/c4-1-2(5)3(6)7/h4H,1H2,(H,6,7)
InChI key
HHDDCCUIIUWNGJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Hydroxy(phenyl)pyruvic acid reductase in Actaea racemosa L.: a putative enzyme in cimicifugic and fukinolic acid biosynthesis.: This study investigates the role of ß-Hydroxypyruvic acid in the biosynthesis of bioactive compounds in Actaea racemosa, highlighting its importance in plant secondary metabolism and potential applications in medicinal chemistry (Jahn and Petersen, 2024).
- The gut microbiota confers protection in the CNS against neurodegeneration induced by manganism.: Research shows the protective role of ß-Hydroxypyruvic acid derivatives produced by gut microbiota against neurodegenerative conditions, providing insights into therapeutic strategies for neurological disorders (Wang et al., 2020).
Biochem/physiol Actions
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service