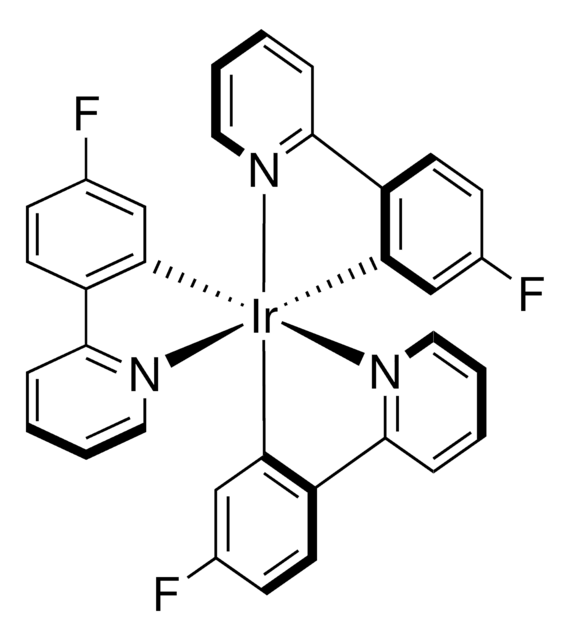
901409
[Ir(dF(Me)ppy)2(dtbbpy)]PF6
Synonym(s):
Iridium(III) bis[2-(2,4-difluorophenyl)-5-methylpyridine-N,C20]-4,40-di-tert-butyl-2,20-bipyridine hexafluorophosphate
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C42H40F10IrN4P
CAS Number:
Molecular Weight:
1013.97
UNSPSC Code:
12161600
NACRES:
NA.22
Recommended Products
form
solid
Quality Level
reaction suitability
core: iridium
reaction type: Photocatalysis
reagent type: catalyst
photocatalyst activation
450 nm
Application
[Ir(dF(Me)ppy)2(dtbbpy)]PF6 is a photocatalyst readily facilitates hydroamination of olefins as well as the decarboxylative arylation and vinylation of carboxylic acids via metallaphotoredox catalysis.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Other Notes
Catalytic intermolecular hydroaminations of unactivated olefins with secondary alkyl amines
Merging photoredox and nickel catalysis: Decarboxylative cross-coupling of carboxylic acids with vinyl halides
Enhanced luminescent iridium(III) complexes bearing aryltriazole cyclometallated ligands
Site-Selective and Stereoselective C-H Alkylations of Carbohydrates via Combined Diarylborinic Acid and Photoredox Catalysis
Merging photoredox and nickel catalysis: Decarboxylative cross-coupling of carboxylic acids with vinyl halides
Enhanced luminescent iridium(III) complexes bearing aryltriazole cyclometallated ligands
Site-Selective and Stereoselective C-H Alkylations of Carbohydrates via Combined Diarylborinic Acid and Photoredox Catalysis
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Adam Noble et al.
Journal of the American Chemical Society, 137(2), 624-627 (2014-12-19)
Decarboxylative cross-coupling of alkyl carboxylic acids with vinyl halides has been accomplished through the synergistic merger of photoredox and nickel catalysis. This new methodology has been successfully applied to a variety of α-oxy and α-amino acids, as well as simple
Strongly Blue Luminescent Cationic Iridium(III) Complexes with an Electron-Rich Ancillary Ligand: Evaluation of Their Optoelectronic and Electrochemiluminescence Properties.
Ladouceur S, et al.
European Journal of Inorganic Chemistry, 2013(30), 5329-5343 (2013)
Andrew J Musacchio et al.
Science (New York, N.Y.), 355(6326), 727-730 (2017-02-18)
The intermolecular hydroamination of unactivated alkenes with simple dialkyl amines remains an unsolved problem in organic synthesis. We report a catalytic protocol for efficient additions of cyclic and acyclic secondary alkyl amines to a wide range of alkyl olefins with
Sébastien Ladouceur et al.
Inorganic chemistry, 50(22), 11514-11526 (2011-10-04)
Herein we report the synthesis of 4-aryl-1-benzyl-1H-1,2,3-triazoles (atl), made via "Click chemistry" and their incorporation as cyclometallating ligands into new heteroleptic iridium(III) complexes containing diimine (N(^)N) ancillary ligands 2,2'-bipyridine (bpy) and 4,4'-di-tert-butyl-2,2'-bipyridine (dtBubpy). Depending on decoration, these complexes emit from
Victoria Dimakos et al.
Journal of the American Chemical Society, 141(13), 5149-5153 (2019-03-23)
Diphenylborinic acid serves as a cocatalyst for site- and stereoselective C-H alkylation reactions of carbohydrates under photoredox conditions using quinuclidine as the hydrogen atom transfer mediator. Products arising from selective abstraction of the equatorial hydrogens of cis-1,2-diol moieties, followed by
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)
![[Ir{dFCF3ppy}2(bpy)]PF6](/deepweb/assets/sigmaaldrich/product/structures/180/924/79119ac4-7d62-429d-b23d-a14c012c6050/640/79119ac4-7d62-429d-b23d-a14c012c6050.png)
![[Ir(dtbbpy)(ppy)2]PF6](/deepweb/assets/sigmaaldrich/product/structures/158/329/2544d673-d267-4aa1-8f46-2652aad4bfa0/640/2544d673-d267-4aa1-8f46-2652aad4bfa0.png)
![[Ir(dFCF3ppy)2-(5,5’-dCF3bpy)]PF6 ≥95%](/deepweb/assets/sigmaaldrich/product/structures/422/901/e00f3148-fb86-4f94-9e79-21d064c3f327/640/e00f3148-fb86-4f94-9e79-21d064c3f327.png)
![Ir[dFFppy]2-(4,4′-dCF3bpy)PF6 ≥95%](/deepweb/assets/sigmaaldrich/product/structures/816/772/b116c17c-e6b2-4c95-be64-45a5a851d823/640/b116c17c-e6b2-4c95-be64-45a5a851d823.png)

![[4,4′-Bis(1,1-dimethylethyl)-2,2′-bipyridine] nickel (II) dichloride](/deepweb/assets/sigmaaldrich/product/structures/471/091/6faa29b1-bf8a-4d87-90b2-4cc55e082620/640/6faa29b1-bf8a-4d87-90b2-4cc55e082620.png)


![[Ir(p-F(Me)ppy)2-(4,4′-dtbbpy)]PF6 ≥95%](/deepweb/assets/sigmaaldrich/product/structures/231/079/a5445824-9d4b-4c84-9c5f-f3acbcc75fd4/640/a5445824-9d4b-4c84-9c5f-f3acbcc75fd4.png)
![Ir[dF(t-Bu)-ppy]3](/deepweb/assets/sigmaaldrich/product/structures/254/294/d0fb19e5-05b2-4c1b-990b-a99fa60b3e73/640/d0fb19e5-05b2-4c1b-990b-a99fa60b3e73.png)
![Tris[2-phenylpyridinato-C2,N]iridium(III) 97%](/deepweb/assets/sigmaaldrich/product/structures/167/234/658d0b76-d31d-4fd5-8041-e04e207227c9/640/658d0b76-d31d-4fd5-8041-e04e207227c9.png)