534579
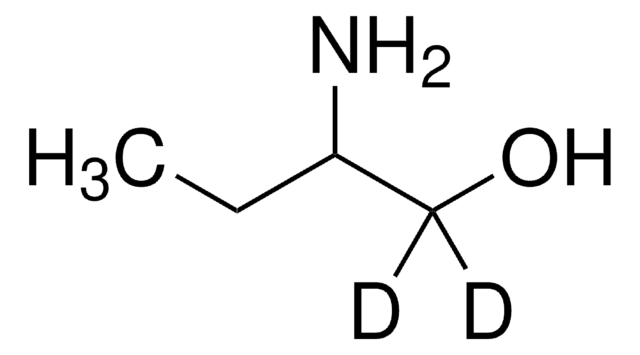
(R)-(−)-2-Amino-1-pentanol
97%
Synonym(s):
D-Norvalinol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3(CH2)2CH(NH2)CH2OH
CAS Number:
Molecular Weight:
103.16
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
optical activity
[α]20/D −17°, c = 1 in chloroform
mp
44-48 °C (lit.)
SMILES string
CCC[C@@H](N)CO
InChI
1S/C5H13NO/c1-2-3-5(6)4-7/h5,7H,2-4,6H2,1H3/t5-/m1/s1
InChI key
ULAXUFGARZZKTK-RXMQYKEDSA-N
Application
(R)-(-)-2-Amino-1-pentanol can be used as a chiral building block to prepare:
- A key intermediate, (R)-N-(p-toluenesulfonyl)-2-propylaziridine, which is utilized in the total synthesis of (R)-1-(benzofuran-2-yl)-2-propylaminopentane.
- (R)-N-benzyloxycarbonyl-aminoaldehydes as potential substrates for dihydroxyacetone phosphate (DHAP)-dependent aldolases.
- (R)-2-((2-Aminoquinazolin-4-yl)amino)pentan-1-ol as a potent dual toll-like receptor and modulator.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
204.8 °F - closed cup
Flash Point(C)
96 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
2, 4-diaminoquinazolines as dual toll-like receptor (TLR) 7/8 modulators for the treatment of hepatitis B virus
Embrechts W, et al.
Journal of Medicinal Chemistry, 61(14), 6236-6246 (2018)
Dihydroxyacetone phosphate aldolase catalyzed synthesis of structurally diverse polyhydroxylated pyrrolidine derivatives and evaluation of their glycosidase inhibitory properties
Calveras J, et al.
Chemistry?A European Journal , 15(30), 7310-7328 (2009)
Enantioselective synthesis and absolute configuration of (-)-1-(benzofuran-2-yl)-2-propylaminopentane,((-)-BPAP), a highly potent and selective catecholaminergic activity enhancer
Oka T, et al.
Bioorganic & Medicinal Chemistry, 9(5), 1213-1219 (2001)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service