All Photos(2)
About This Item
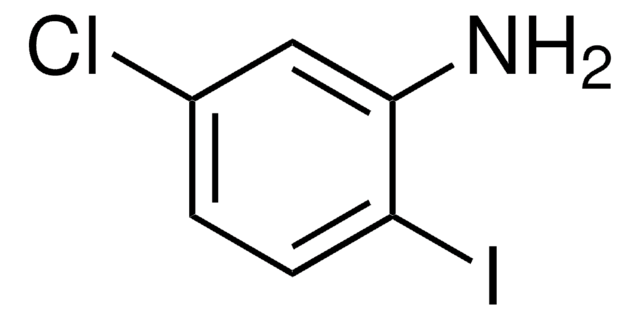
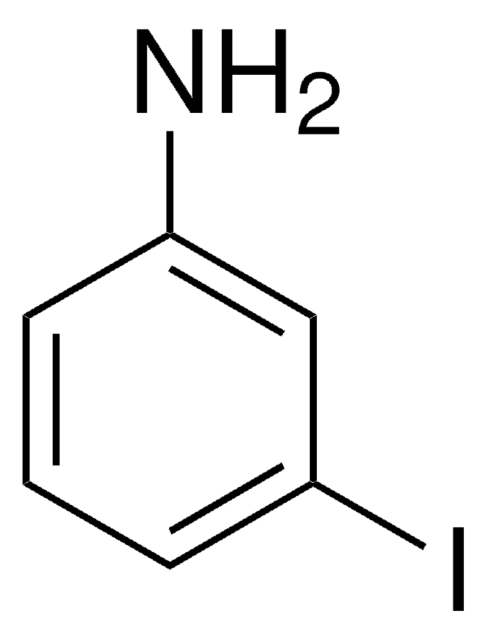
Linear Formula:
ClC6H3(I)NH2
CAS Number:
Molecular Weight:
253.47
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
39-43 °C (lit.)
functional group
chloro
iodo
SMILES string
Nc1ccc(Cl)cc1I
InChI
1S/C6H5ClIN/c7-4-1-2-6(9)5(8)3-4/h1-3H,9H2
InChI key
FLEJOBRWKBPUOX-UHFFFAOYSA-N
Related Categories
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
>228.2 °F - closed cup
Flash Point(C)
> 109 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Quinazolines. VI. Synthesis of 2,4-diaminoquinazolines from anthranilonitriles.
A Rosowsky et al.
Journal of medicinal chemistry, 13(5), 882-886 (1970-09-01)
Stereoselective Synthesis of (E)-3-(Methoxycarbonyl) methylene-1, 3-dihydroindol-2-ones by Palladium-Catalyzed Oxidative Carbonylation of 2-Ethynylanilines.
Gabriele B, et al.
European Journal of Organic Chemistry, 24, 4607-4613 (2001)
Wing S Cheung et al.
The Journal of organic chemistry, 70(9), 3741-3744 (2005-04-23)
[reaction: see text] An efficient and versatile method for stereoselective synthesis of (E)-3,3-(diarylmethylene)indolinones by a palladium-catalyzed tandem Heck-carbocyclization/Suzuki-coupling sequence is presented. Factors influencing yield and selectivity, namely catalyst, coordinating ligand, and solvent, are detailed.
Regioselective iodination of aryl amines using 1, 4-dibenzyl-1, 4-diazoniabicyclo [2.2. 2] octane dichloroiodate in solution and under solvent-free conditions.
Alikarami M, et al.
Bulletin of the Chemical Society of Ethiopia, 29(1), 157-162 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service