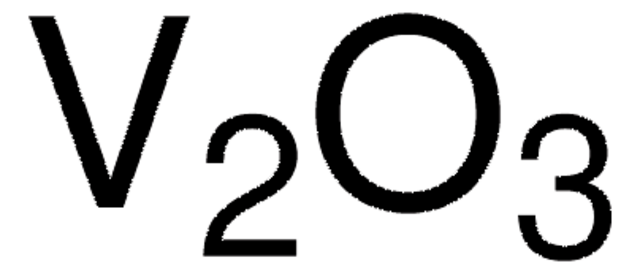215066
Gallium(III) oxide
≥99.99% trace metals basis
Synonym(s):
Gallium trioxide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
Ga2O3
CAS Number:
Molecular Weight:
187.44
EC Number:
MDL number:
UNSPSC Code:
12352303
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
≥99.99% trace metals basis
form
(crystalline powder)
reaction suitability
reagent type: catalyst
core: gallium
density
5.88 g/mL at 25 °C
SMILES string
O=[Ga]O[Ga]=O
InChI
1S/2Ga.3O
InChI key
QZQVBEXLDFYHSR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Gallium(III) oxide (Ga2O3) is a wide band gap semiconductor that belongs to a family of transparent semiconducting oxides (TSO). It can form different polymorphs such as α-,β-, γ-, δ-, and ε-. Polycrystalline and nanocrystalline Ga2O3 can be prepared using several methods such as chemical vapor deposition, thermal vaporization, and sublimation, molecular beam epitaxy, melt growth, etc. It is widely used as a functional material in various applications including optoelectronics, chemical sensors, catalysis, semiconductor devices, field-effect transistors, and many others.
Application
Ga2O3 is widely used as a host material for the fabrication of electroluminescent devices. For example, europium-doped Ga2O3 thin films can be used as a light-emitting layer to fabricate an optically transparent electroluminescent device.
Due to its distinct optical and electrical properties like moderate conductivity and high laser damage threshold, Ga2O3 can be used in laser-driven electron accelerators, low-loss plasmonics, and Si-based dielectric laser accelerators.
It can also be used as an effective catalyst for the dehydrogenation of propane to propene.
Due to its distinct optical and electrical properties like moderate conductivity and high laser damage threshold, Ga2O3 can be used in laser-driven electron accelerators, low-loss plasmonics, and Si-based dielectric laser accelerators.
It can also be used as an effective catalyst for the dehydrogenation of propane to propene.
Starting material for the preparation of Sr2CuGaO3S, an example of a rare square pyramidal gallium.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Vladimir N Sigaev et al.
Nanoscale, 5(1), 299-306 (2012-11-21)
Nanoparticles in amorphous oxides are a powerful tool for embedding a wide range of functions in optical glasses, which are still the best solutions in several applications in the ever growing field of photonics. However, the control of the nanoparticle
Naoya Kumagai et al.
Chemical communications (Cambridge, England), 47(6), 1884-1886 (2010-12-07)
Using the Rh(3+) ion (Rh d(6)) in a regular octahedral coordination, which forms fully occupied t(2g)(6) and empty e(g)(0) as a result of ligand-field splitting, we demonstrated that Rh-doped ZnGa(2)O(4) had midgap states created by t(2g)(6) and e(g)(0) that had
Bipin Pandey et al.
Langmuir : the ACS journal of surfaces and colloids, 28(38), 13705-13711 (2012-09-01)
This paper reports the formation of self-organized nanoporous gallium oxide by anodization of solid gallium metal. Because of its low melting point (ca. 30 °C), metallic gallium can be shaped into flexible structures, permitting the fabrication of nanoporous anodic oxide
Baoxiu Zhao et al.
Journal of environmental sciences (China), 24(4), 774-780 (2012-08-17)
Perfluorooctanoic acid (PFOA) is a new-found hazardous persistent organic pollutant, and it is resistant to decomposition by hydroxyl radical (HO*) due to its stable chemical structure and the high electronegativity of fluorine. Photocatalytic reduction of PFOA with beta-Ga2O3 in anoxic
G Wilma Busser et al.
ChemSusChem, 5(11), 2200-2206 (2012-10-24)
The deposition of hydrogen evolution sites on photocatalysts is a crucial step in the multistep process of synthesizing a catalyst that is active for overall photocatalytic water splitting. An alternative approach to conventional photodeposition was developed, applying the photocatalytic reforming
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service