All Photos(1)
About This Item
Empirical Formula (Hill Notation):
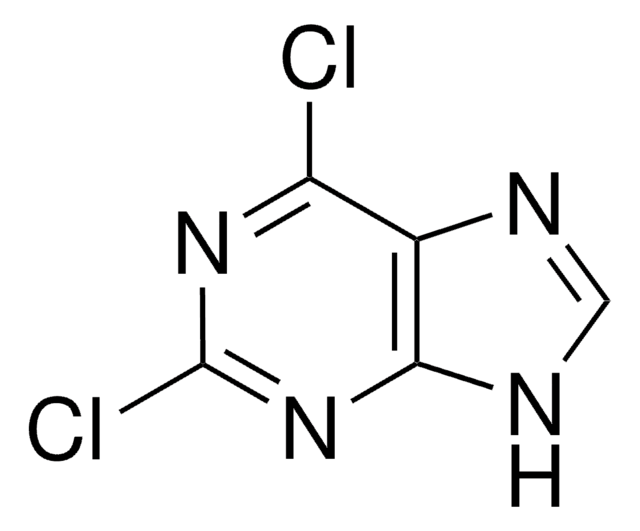
C5H3ClN4
CAS Number:
Molecular Weight:
154.56
Beilstein:
5774
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99%
form
powder
mp
>300 °C (dec.) (lit.)
solubility
DMF: soluble 5%, clear, colorless to yellow
functional group
chloro
SMILES string
Clc1ncnc2[nH]cnc12
InChI
1S/C5H3ClN4/c6-4-3-5(9-1-7-3)10-2-8-4/h1-2H,(H,7,8,9,10)
InChI key
ZKBQDFAWXLTYKS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The acid-catalyzed reaction of 6-chloropurine with 3,4-di-O-acetyl-D-xylal has been investigated.
Application
6-Chloropurine has been used in the preparation of 9-alkylpurines via alkylation with various substituted alkyl halides in DMSO. It was also used in the preparation of 6-succinoaminopurine.
Signal Word
Warning
Hazard Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of 6-succinoaminopurine.
C E CARTER
The Journal of biological chemistry, 223(1), 139-146 (1956-11-01)
Heterocyclic N-glycosides-V: Synthesis of unsaturated N-glycosides from 6-chloropurine and derivatives of d-xylal and l-arabinal. A conformational NMR study.
Fuertes M, et al.
Tetrahedron, 26(20), 4823-4837 (1970)
Synthesis of Potential Anticancer Agents. XXVI. The Alkylation of 6-Chloropurine2.
Montgomery JA and Temple Jr C.
Journal of the American Chemical Society, 83(3), 630-635 (1961)
Prashantha Gunaga et al.
The Journal of organic chemistry, 69(9), 3208-3211 (2004-04-24)
Novel thioiso pyrimidine and purine nucleosides substituted with exocyclic methylene have been synthesized, starting from D-xylose. The glycosyl donor 14 was synthesized from D-xylose, using cyclization of dimesylate 10 with sodium sulfide as a key step. Cyclization proceeded in pure
Michal Sála et al.
Bioorganic & medicinal chemistry letters, 22(5), 1963-1968 (2012-02-09)
We report on the synthesis and the study of the structure-activity relationship of novel 9-norbornyl-6-chloropurine derivatives, which exert selective antiviral activity on the replication of Coxsackievirus B3. In particular, the synthetic approaches towards norbornyl derivatives bearing diverse side chains were
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service