80126
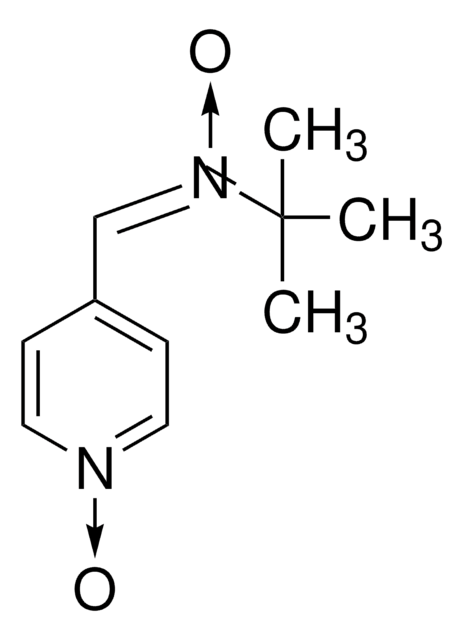
N-tert-Butyl-α-phenylnitrone
for ESR-spectroscopy
Synonym(s):
N-Benzylidene-tert-butylamine N-oxide, PBN, Phenyl N-t-butylnitrone
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5CH=N(O)C(CH3)3
CAS Number:
Molecular Weight:
177.24
Beilstein:
2044028
EC Number:
MDL number:
UNSPSC Code:
12000000
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
grade
for ESR-spectroscopy
Quality Level
Assay
≥99.5% (HPLC)
form
powder
mp
72-74 °C
73-74 °C (lit.)
solubility
chloroform: 50 mg/mL, clear, colorless
storage temp.
−20°C
SMILES string
CC(C)(C)[N+](\[O-])=C\c1ccccc1
InChI
1S/C11H15NO/c1-11(2,3)12(13)9-10-7-5-4-6-8-10/h4-9H,1-3H3/b12-9-
InChI key
IYSYLWYGCWTJSG-XFXZXTDPSA-N
Looking for similar products? Visit Product Comparison Guide
Application
N-tert-Butyl-a-phenylnitrone was used as spin trapping agent during measurement of scavenging rate constant of carotenoid using EPR spin-trapping technique. This reagent helps in obtaining a six-line ESR spectrum and hyperfine coupling constants, confirming the presence of carbon-based radical in uric acid and peroxynitrite, using electron spin resonance spectroscopy and liquid chromatography mass spectrometry.
Biochem/physiol Actions
N-tert-butyl-α-phenylnitrone (PBN) is a commonly used free-radical spin trap.
N-tert-butyl-α-phenylnitrone (PBN) is a commonly used free-radical spin trap. It has been shown to reduce the number of emboli-induced cerebral microinfarctions in the rabbit cortex and prevent neoplasia by its radical scavenging activity and its ability to inhibit cyclooxygenase-2 activity. Reported to inhibit the induction of nitric oxide synthase (iNOS), thereby preventing the overproduction of nitric oxide (NO). PBN in a dose of 100 mg/kg i.p. reduced necrosis of the substantia nigra, pars reticulate in flurothyl-induced status epilepticus in rats. It protects against some types of post-trauma epileptogenic events in an animal model of epilepsy. The lethal dose of PBN in rats was found to be approximately 100 mg/100 g body weight (0.564 mmol/100Å g).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Antioxidant and redox properties of supramolecular complexes of carotenoids with beta-glycyrrhizic acid.
Polyakov NE
Free Radical Biology & Medicine, 40(10), 1804-1809 (2006)
Radicals in the reaction between peroxynitrite and uric acid identified by electron spin resonance spectroscopy and liquid chromatography mass spectrometry.
Imaram W
Free Radical Biology & Medicine, 49(2), 275-281 (2010)
Jian-Jun Wen et al.
Journal of the American College of Cardiology, 55(22), 2499-2508 (2010-06-01)
The purpose of this study was to determine the pathological importance of oxidative stress-induced injurious processes in chagasic heart dysfunction. Trypanosoma cruzi-induced inflammatory pathology and a feedback cycle of mitochondrial dysfunction and oxidative stress may contribute to Chagas disease. Sprague-Dawley
Fanny Choteau et al.
The Journal of organic chemistry, 77(2), 938-948 (2011-12-23)
A novel series of α-phenyl-N-tert-butyl nitrone derivatives, bearing a hydrophobic chain on the aromatic ring and three hydroxyl functions on the tert-butyl group, was synthesized through a short and convenient synthetic route based on a one-pot reduction/condensation of tris(hydroxymethyl)nitromethane with
Jiwon Yang et al.
Journal of neurochemistry, 124(4), 523-535 (2012-12-04)
Oxidative stress after stroke is associated with the inflammatory system activation in the brain. The complement cascade, especially the degradation products of complement component 3, is a key inflammatory mediator of cerebral ischemia. We have shown that pro-inflammatory complement component
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service