803480
PDPH (3-(2-pyridyldithio)propionyl hydrazide)
Synonym(s):
3-(2-Pyridyldithio)propionate hydrazide, 3-[(2-Pyridyl)dithio]propionohydrazide
About This Item
Recommended Products
Assay
≥95%
Quality Level
form
powder
mol wt
229.32
reaction suitability
reagent type: cross-linking reagent
storage condition
desiccated
solubility
DMSO or DMF: soluble
functional group
hydrazide
shipped in
ambient
storage temp.
2-8°C
SMILES string
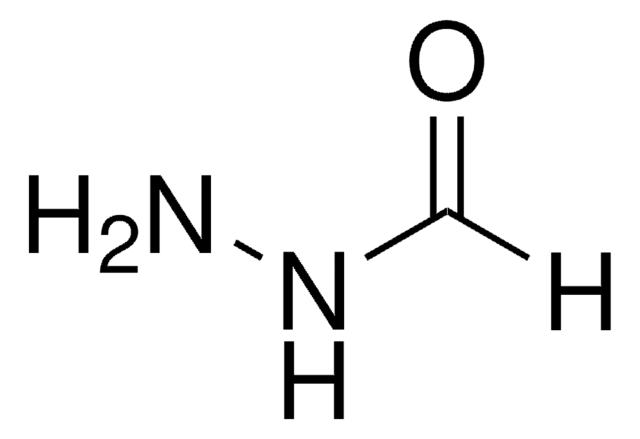
NNC(CCSSC1=NC=CC=C1)=O
InChI
1S/C8H11N3OS2/c9-11-7(12)4-6-13-14-8-3-1-2-5-10-8/h1-3,5H,4,6,9H2,(H,11,12)
InChI key
NITXODYAMWZEJY-UHFFFAOYSA-N
General description
Features and Benefits
- Reactive groups: pyridyldisulfide and hydrazide
- Reactive toward: sulfhydryl groups and carbonyl (aldehyde) groups
- Short (9.2A), sulfhydryl-to-aldehyde crosslinker with disulfide bond spacer arm (cleavable)
- Pyridyldithiol group results in attachment to sulfhydryls via disulfide bond, which can be cleaved with DTT, TCEP or other reducing agents
- Hydrazide group conjugates to oxidized sugars of glycoproteins and carbohydrates
- Use sodium meta-periodate to oxidize glycosylation (e.g., sialic acid) to reactive aldehyde groups
- Use with EDC to conjugate primary amine of hydrazide group to carboxyl groups
Caution
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![LC-SPDP (succinimidyl 6-[3(2-pyridyldithio)propionamido]hexanoate)](/deepweb/assets/sigmaaldrich/product/structures/300/586/d95fd80c-e201-4b0b-8aee-31e109c2ff41/640/d95fd80c-e201-4b0b-8aee-31e109c2ff41.png)





![Sulfo-LC-SPDP (sulfosuccinimidyl 6-[3′-(2-pyridyldithio)propionamido]hexanoate)](/deepweb/assets/sigmaaldrich/product/structures/266/633/e2a263be-4bd3-4fcf-89c4-75b5e2bd829c/640/e2a263be-4bd3-4fcf-89c4-75b5e2bd829c.png)