All Photos(1)
About This Item
Linear Formula:
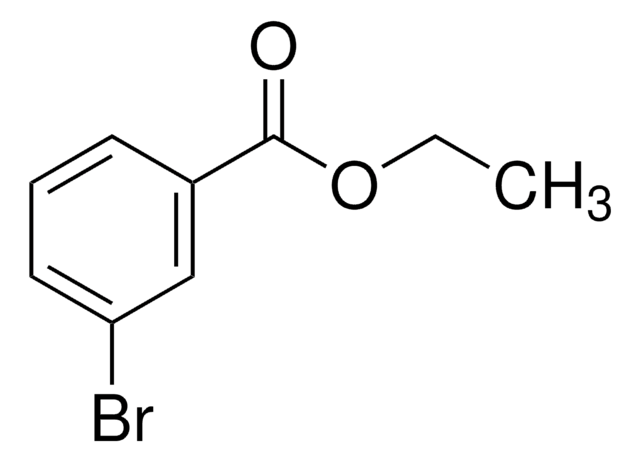
BrC6H4CO2CH3
CAS Number:
Molecular Weight:
215.04
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
bp
127-128 °C/15 mmHg (lit.)
mp
31-33 °C (lit.)
SMILES string
COC(=O)c1cccc(Br)c1
InChI
1S/C8H7BrO2/c1-11-8(10)6-3-2-4-7(9)5-6/h2-5H,1H3
InChI key
KMFJVYMFCAIRAN-UHFFFAOYSA-N
General description
Methyl 3-bromobenzoate is an aryl bromide. It undergoes stereoconvergent cross-coupling with potassium trifluoro(1-phenylethyl)borate to form 1,1-diarylethane derivative. The Negishi cross-coupling reaction between methyl 3-bromobenzoate and diarylzinc reagents in the presence of a palladium catalyst has been reported. Methyl-3-bromobenzoate can be converted into the corresponding benzonitrile using dichloro[bis{1-(dicyclohexylphosphanyl)piperidine}]palladium as a C-C cross-coupling catalyst and K4[Fe(CN)6] as a cyanating agent.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Negishi cross-coupling reaction catalyzed by an aliphatic, phosphine based pincer complex of palladium. biaryl formation via cationic pincer-type PdIV intermediates.
Gerber R, et al.
Dalton Transactions, 40(35), 8996-9003 (2011)
Roman Gerber et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(10), 2978-2986 (2012-02-03)
Dichloro[bis{1-(dicyclohexylphosphanyl)piperidine}]palladium [(P{(NC(5)H(10))(C(6)H(11))(2)})(2)PdCl(2)] (1) is a highly active and generally applicable C-C cross-coupling catalyst. Apart from its high catalytic activity in Suzuki, Heck, and Negishi reactions, compound 1 also efficiently converted various electronically activated, nonactivated, and deactivated aryl bromides, which may
John C Tellis et al.
Science (New York, N.Y.), 345(6195), 433-436 (2014-06-07)
The routine application of C(sp3)-hybridized nucleophiles in cross-coupling reactions remains an unsolved challenge in organic chemistry. The sluggish transmetalation rates observed for the preferred organoboron reagents in such transformations are a consequence of the two-electron mechanism underlying the standard catalytic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service