All Photos(1)
About This Item
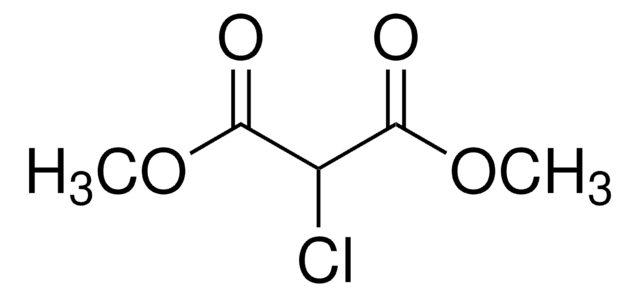
Linear Formula:
ClCH2CON(OCH3)CH3
CAS Number:
Molecular Weight:
137.56
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
39-41 °C (lit.)
storage temp.
2-8°C
SMILES string
CON(C)C(=O)CCl
InChI
1S/C4H8ClNO2/c1-6(8-2)4(7)3-5/h3H2,1-2H3
InChI key
SCOJKGRNQDKFRP-UHFFFAOYSA-N
Related Categories
General description
2-Chloro-N-methoxy-N-methylacetamide is a Weinreb amide.
Application
2-Chloro-N-methoxy-N-methylacetamide may be used in the preparation of:
- 2-heptyl-3-hydroxy-4(1H)-quinolone (Pseudomonas quinolone signal or PQS) and structurally related 2-alkyl-4-quinolones having biological activity
- 2-(benzo[d]thiazol-2-ylsulfonyl)-N-methoxy-N-methylacetamide
- α-chloro-ketone, starting reagent for the one-pot synthesis of 2-heptyl-3-hydroxyl-4(1H)-quinolone (PQS), signalling molecule in the quorum sensing of Pseudomonas aeruginosa
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
219.2 °F - closed cup
Flash Point(C)
104 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
New Reagent for Convenient Access to the a, ?-Unsaturated N-Methoxy-N-methyl-amide Functionality by a Synthesis Based on the Julia Olefination Protocol.
Narayana Manjunath B, et al
European Journal of Organic Chemistry, 12, 2851-2855 (2006)
James T Hodgkinson et al.
Organic & biomolecular chemistry, 9(1), 57-61 (2010-10-23)
Expedient syntheses of Pseudomonas quinolone signal (PQS) and related structural analogues using microwave and flow methods are reported.
James T Hodgkinson et al.
Nature protocols, 7(6), 1184-1192 (2012-05-29)
An optimized procedure for the efficient preparation of 2-heptyl-3-hydroxy-4(1H)-quinolone (Pseudomonas quinolone signal or PQS) and a diverse range of structurally related 2-alkyl-4-quinolones with biological activity is presented. The two-step synthesis begins with the formation of α-chloro ketones by the coupling
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service