15404
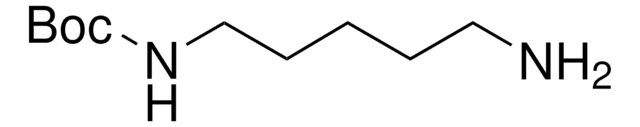
N-Boc-1,4-butanediamine
≥97.0% (GC/NT)
Synonym(s):
N-Boc-1,4-diaminobutane, tert-Butyl N-(4-aminobutyl)carbamate
About This Item
Recommended Products
Quality Level
Assay
≥97.0% (GC/NT)
reaction suitability
reagent type: cross-linking reagent
refractive index
n20/D 1.460
density
0.984 g/mL at 20 °C (lit.)
functional group
Boc
amine
SMILES string
NCCCCNC(OC(C)(C)C)=O
InChI
1S/C9H20N2O2/c1-9(2,3)13-8(12)11-7-5-4-6-10/h4-7,10H2,1-3H3,(H,11,12)
InChI key
ZFQWJXFJJZUVPI-UHFFFAOYSA-N
Application
- Carboxy-Silane Coated Iron Oxide Nanoparticles: Details the application of N-Boc-1,4-butanediamine in modifying iron oxide nanoparticles for imaging and drug delivery (D Stanicki, S Boutry, S Laurent, et al., 2014). Access the article.
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
228.2 °F - closed cup
Flash Point(C)
109.0 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Mono-Boc-protected diamines are versatile building blocks for chemical synthesis. Their production is a lot more challenging than the simple reaction scheme might imply, because the Boc-anhydride reagent cannot differentiate between the two identical amino moieties in the substrate.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service