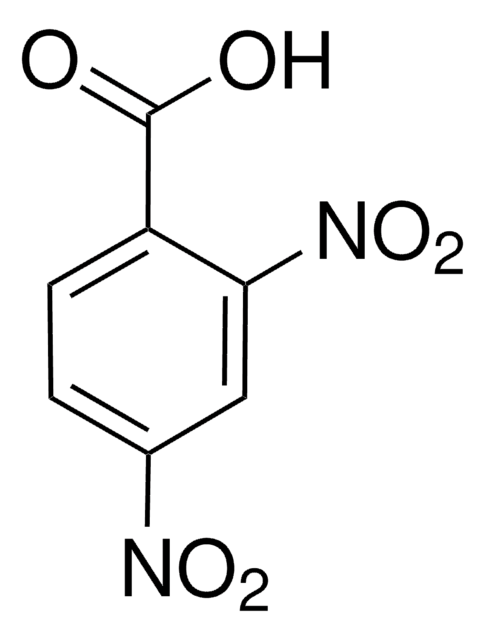
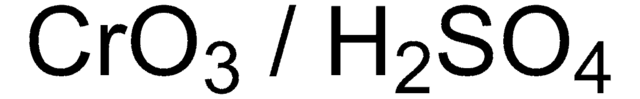156272
3,5-Dinitrobenzoyl chloride
≥96.5%
Synonym(s):
DNBC, NSC 2697
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(O2N)2C6H3COCl
CAS Number:
Molecular Weight:
230.56
Beilstein:
990249
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
vapor density
7.6 (vs air)
Quality Level
Assay
≥96.5%
form
solid
bp
196 °C/11 mmHg (lit.)
mp
68-69 °C (lit.)
SMILES string
[O-][N+](=O)c1cc(cc(c1)[N+]([O-])=O)C(Cl)=O
InChI
1S/C7H3ClN2O5/c8-7(11)4-1-5(9(12)13)3-6(2-4)10(14)15/h1-3H
InChI key
NNOHXABAQAGKRZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Crystals of 3,5-dinitrobenzoyl chloride (DNBC) belong to the orthorhombic crystal system and space group Pna21. It is widely used reagent for the detection of alcohols.
Application
3,5-Dinitrobenzoyl chloride may be used for the quantitative estimation of hydroxyl groups in pyridine. It may be used in the synthesis of following:
3,5-Dinitrobenzoyl chloride may also be used as derivatizing agent for the following:
- 3,5-diamino-N-cyclopropylbenzamide
- 3,5-dinitro-N-cyclopropylbenzamide
- 4-(3,5-dinitrophenyl)-6-(dipropan-2-ylamino)-2H-1,3,5-thiadiazine-2-thione4
3,5-Dinitrobenzoyl chloride may also be used as derivatizing agent for the following:
- biogenic amines present in fermented foods, which can be determined using liquid chromatography method(LC)
- polyamines, present in biological fluids, which can be determined using high performance liquid chromatography(HPLC)
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rapid Determination of Organic Hydroxyl Groups with 3, 5,-Dinitrobenzoyl Chloride.
Robinson Jr WT, et al.
Analytical Chemistry, 33(8), 1030-1034 (1961)
Synthesis and characterization of aromatic poly (amides) based on 3, 5-diamino-N-cyclopropylbenzamide.
Tundidor-Camba A, et al.
Royal Society of Chemistry Advances, 5(29), 23057-23066 (2015)
S Wongyai et al.
Biomedical chromatography : BMC, 2(6), 254-257 (1988-11-01)
Analytical methods are described for the quantitative determination of putrescine, cadaverine, spermidine, spermine and the acetylated derivatives of spermidine and spermine in biological fluids using pre-column derivatization with either benzoyl chloride or 3,5-dinitrobenzoyl chloride, which were added to each sample
3, 5-Dinitrobenzoyl chloride.
Wang HY, et al.
Acta Crystallographica Section E, Structure Reports Online, 65(10), o2460-o2460 (2009)
A Markowska et al.
Roczniki Akademii Medycznej w Bialymstoku (1995), 42(1), 129-140 (1997-01-01)
A carbocyclic analogue of distamycin was obtained, in which the N-methylpyrrole rings were substituted by disubstituted benzene rings. Additionally, N-chloro- or N-bromoacetyl groups, displaying alkylating properties, were introduced. The synthesis, starting from 3,5-dinitrobenzoyl chloride, consisted of five stages.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service