All Photos(1)
About This Item
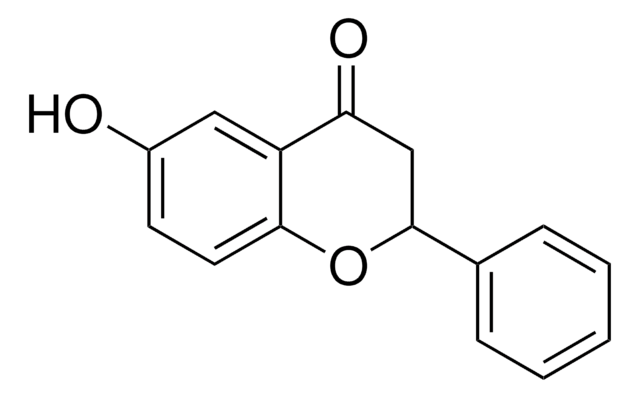
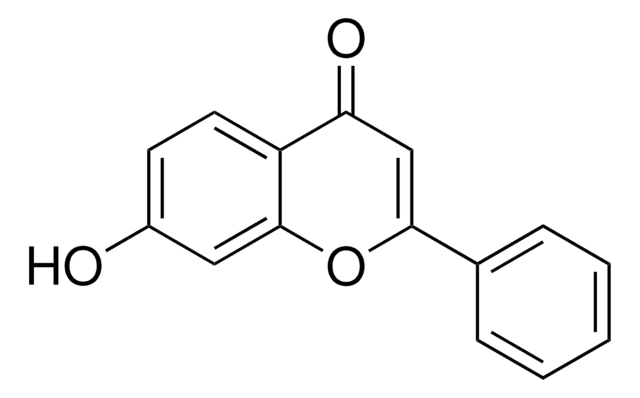
Empirical Formula (Hill Notation):
C15H10O3
CAS Number:
Molecular Weight:
238.24
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
234-236 °C (lit.)
SMILES string
Oc1ccc2OC(=CC(=O)c2c1)c3ccccc3
InChI
1S/C15H10O3/c16-11-6-7-14-12(8-11)13(17)9-15(18-14)10-4-2-1-3-5-10/h1-9,16H
InChI key
GPZYYYGYCRFPBU-UHFFFAOYSA-N
Gene Information
rat ... Ar(24208) , Gabra2(29706)
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Structure of 6-hydroxyflavone.
Seetharaman J and Rajan SS.
Acta Crystallographica Section C, Structural Chemistry, 48(9), 1714-1715 (1992)
Xing Wang et al.
PloS one, 10(3), e0116409-e0116409 (2015-03-20)
Inflammatory responses by kidney mesangial cells play a critical role in the glomerulonephritis. The anti-inflammatory potential of nineteen mono-, di- and polyhydroxylated flavones including fisetin, quercetin, morin, tricetin, gossypetin, apigenin and myricetin were investigated on rat mesangial cells with lipopolysaccharide
Zia Ud Din et al.
Histology and histopathology, 35(10), 1197-1209 (2020-09-11)
In this study, the flavonoid, 6-hydroxyflavone was investigated for its renal protective activity in the cisplatin rat model of nephrotoxicity. Male Sprague-Dawley rats weighing 200-250 g were included in the study. 6-Hydroxyflavone was daily administered at 25 and 50 mg/kg
A novel and facile iodine (III)-mediated approach for C (5)-acetoxylation of 6-hydroxyflavone and 6-hydroxyflavanones.
Prakash O, et al.
Tetrahedron Letters, 45(49), 9065-9067 (2004)
Sourav Das et al.
Journal of biomolecular structure & dynamics, 37(15), 4019-4034 (2018-10-14)
The interaction of 6-hydroxyflavone (6HF) with hen egg white lysozyme (HEWL) has been executed using multi-spectroscopic and computational methods. Steady state fluorescence studies indicated that static quenching mechanism is involved in the binding of 6HF with HEWL, which was further
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service