324906
4,5,6,7-Tetrahydroindole
98%
Synonym(s):
2,3-Tetramethylenepyrrole, Cyclohex[b]pyrrole, NSC 122455
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C8H11N
CAS Number:
Molecular Weight:
121.18
Beilstein:
108853
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
53-57 °C (lit.)
storage temp.
2-8°C
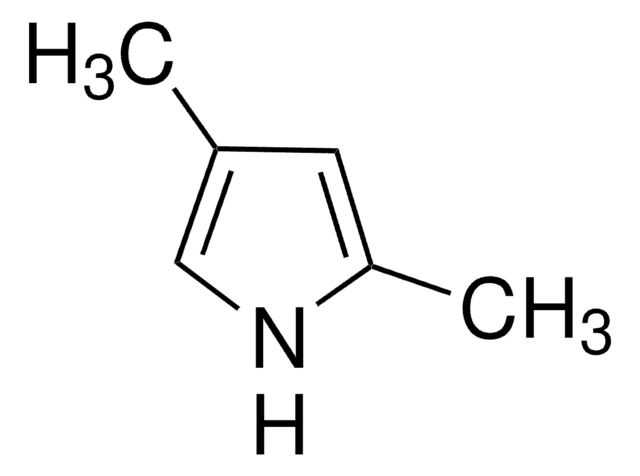
SMILES string
C1CCc2[nH]ccc2C1
InChI
1S/C8H11N/c1-2-4-8-7(3-1)5-6-9-8/h5-6,9H,1-4H2
InChI key
KQBVVLOYXDVATK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4,5,6,7-Tetrahydroindoles, due to their easy aromatization, are good intermediates to synthesize indoles. 4,5,6,7-Tetrahydroindole on condensation with cyanoacetate leads to 1-ethylthio-2-cyano-4,5,6,7-tetrahydrocyclohexa-[c]-3H-pyrrolizin-3-one.
Application
4,5,6,7-Tetrahydroindole was used as reactant in:
- synthesis of ethyl 3-(4,5,6,7-tetrahydroindol-2-yl)-2-propynoate
- preparation of BODIPY dyes
- N-alkylation with chloromethyloxirane
- preparation of hydroindolepropynoate by chemo- and regioselective solvent-free ethynylation
- palladium- and copper-free cross-coupling of halopropynoates
- preparation of carbonylalkenyl indoles via coupling with dicarbonyl compounds
- 1:2 annelation of 4,5,6,7-tetrahydroindole with 1-benzoyl-2-phenylacetylene
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Sobenina, L. N.; et al.
Khim. Geterotsikl. Soedin., 39, 1113-1113 (2003)
Pyrrole-2-dithiocarboxylates: Synthesis of 2-(1-Alkylthio-2-cyanoethenyl) pyrroles.
Sobenina LN, et al.
Tetrahedron, 51(14), 4223-4230 (1995)
Arcadi, A.; et al.
Advanced Synthesis & Catalysis, 348, 331-331 (2006)
Markova, M., V.; et al.
ARKIVOC (Gainesville, FL, United States), 57-57 (2008)
Chemo-and regioselective ethynylation of 4, 5, 6, 7-tetrahydroindoles with ethyl 3-halo-2-propynoates.
Trofimov, B., A.; et al.
Tetrahedron Letters, 49, 3946-3946 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service